Anti-tumor protein and preparation method and application thereof
An anti-tumor and protein technology, applied in the field of anti-lymphoma protein and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
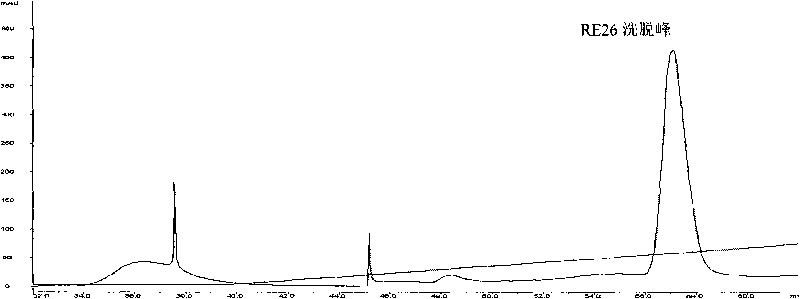
[0063] Example 1 Separation and purification of RE26 protein
[0064] (1) Obtaining of crude extract:
[0065] Take the fresh fruiting body of Rozites emodensis (Berk.) Moser that has been fully expanded, discard the stem, wash the canopy with distilled water, drain, and add 0.02 volume of pH 8.0 mol / l Tris-HCl buffer solution, homogenate at 4°C, adjust the pH of the solution to 8.0 with 1 mol / l NaOH, and gently stir overnight, after centrifugation, filter the supernatant to remove suspended matter to obtain RE26 crude extract.
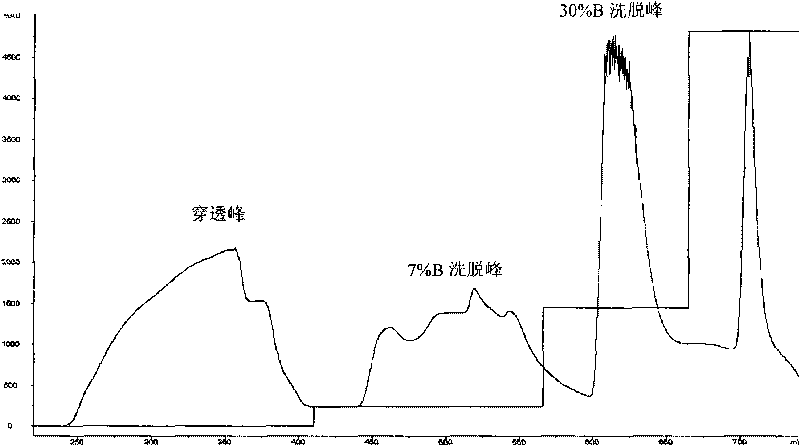
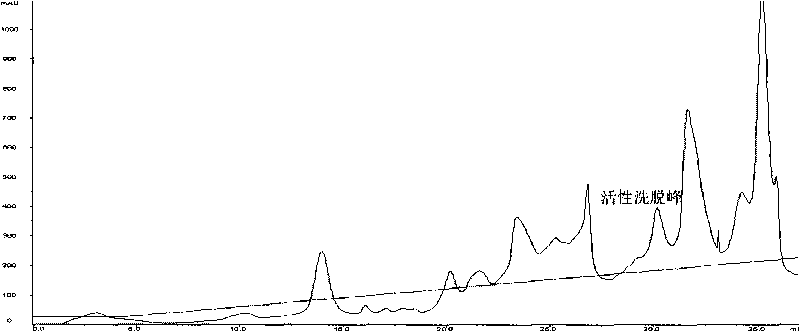
[0066] (2) Purification of RE26: High-purity RE26 protein can be obtained by three ion exchange methods.
[0067] The first ion-exchange chromatography: use the Sepharose Q Fast Flow column (particle diameter 45-165 μ m) that effective column height is 10cm to carry out anion exchange, use the Tris-HCl buffer solution of pH 8.0, 0.02mol / l as mobile phase A, Using 1 mol / l NaCl, pH 8.0, 0.02 mol / l Tris-HCl buffer as the mobile phase B, the linear flow...
Embodiment 2
[0071] Partial physicochemical property research of embodiment two RE26
[0072] (1) SDS-PAGE electrophoresis analysis:
[0073] SDS-PAGE electrophoresis (gel concentration is 12%) is used to identify the purity of the RE26 obtained by the separation of Example 1, and determine the molecular weight. The experimental results are as attached Figure 4 As shown, it shows that the purity of RE26 sample is higher than 95%, and the molecular size of RE26 protein is about 26kD.
[0074] (2) Isoelectric focusing experiment:
[0075] The purified RE26 was desalted using a desalting column and replaced with a pure aqueous solution. Isoelectric focusing was performed on a Bio-Rad thin-layer isoelectric focusing instrument, using pH 3-10 ampholytes to establish a pH gradient. After focusing, compare the isoelectric point Marker to determine the isoelectric point of RE26, which is about 4.3, as shown in the attached Figure 5 shown. At the same time, the purified RE26 also showed a si...
Embodiment 3
[0081] Example 3 The minimum inhibitory concentration MIC of RE26 to various cells 50 Determination of
[0082] MIC of RE26 on various cells 50The value is obtained by measuring the cell viability with the MTT method using the drug after doubling dilution to act on the test cells for 48 hours. Tested cells include: Yac-1 (mouse T lymphoma cells), Raji (human Burkitt's lymphoma), HPC (human hepatocytes), Hepg2 (human liver cancer cells), El-4 (mouse lymphoma cells), MSC (mouse mesenchymal stem cells), ECV-304 (human umbilical vein endothelial cells), Vero-E6 (African green monkey kidney cells), primary spleen lymphocytes of mice, and red blood cells of rats.
[0083] Dissolve RE26 in 1640-calf serum medium to 1 mg / ml, and then use the same medium to double-dilute it 12 times in sequence for use.
[0084] Various cells were formulated into 5 × 10 4 Cell suspension at a concentration of 1 / ml was inoculated in a 96-well plate at 100 μl per well, and each type of cell was inocu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com