Nuclear shell structure mesoporous heavy metal ion adsorbent capable of magnetic separation and preparation method thereof
A heavy metal ion and core-shell structure technology, applied in the field of mesoporous adsorption materials, can solve the problems of low efficiency, complicated operation and high cost of filtration separation and centrifugal separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
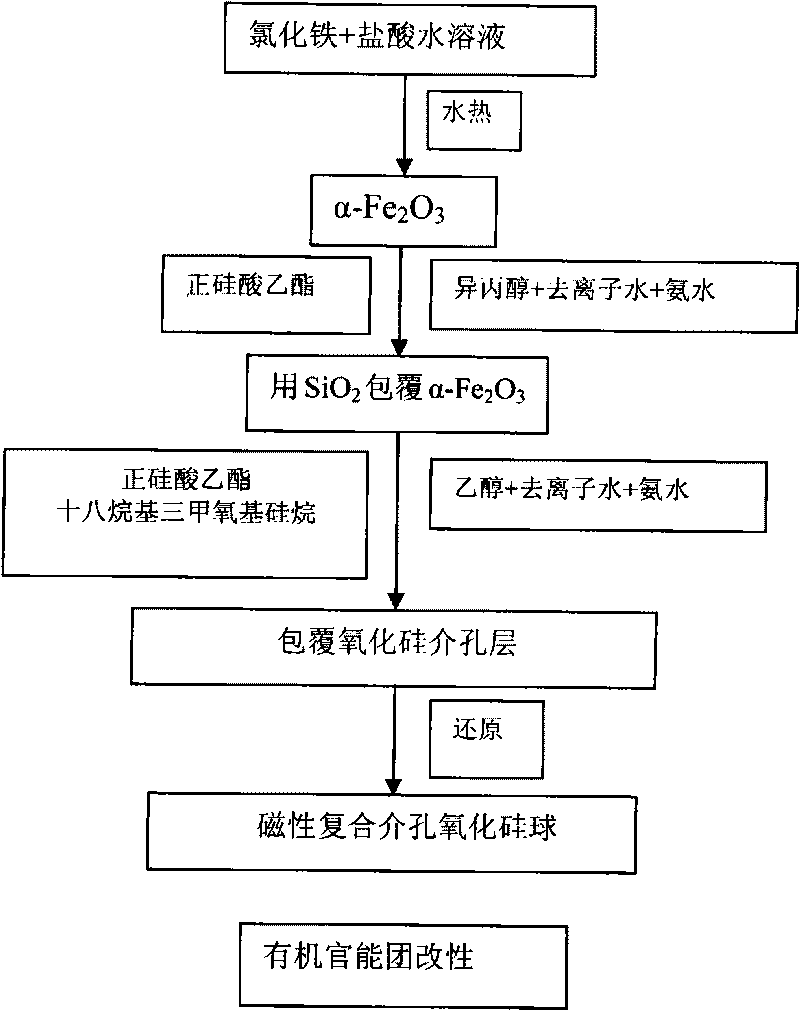
[0029] 1. Preparation of core-shell structure mesoporous heavy metal ion adsorbent (1)
[0030] (1) Synthesis of α-Fe 2 o 3 particles
[0031] Dissolve ferric chloride in deionized water at a concentration of 2.0×10 -2 M, add hydrochloric acid solution, its concentration is: 1×10 -3 M, hydrothermal treatment, the temperature is 100°C, the time is 24h, centrifuged, washed several times with deionized water.
[0032] (2) Coating dense silicon oxide intermediate layer
[0033] Disperse α-iron oxide particles in a mixed solution of isopropanol, deionized water and ammonia water, add tetraethyl orthosilicate, and its concentration range is: 1×10 -3 ~1×10 -2 M, stirred for 5-10 hours, centrifuged and washed several times with ethanol.
[0034] (3) Coated with silicon oxide mesoporous layer
[0035] Disperse the particles coated with the dense silicon oxide intermediate layer in a mixed solution of ethanol, deionized water, and ammonia water, and add a mixed solution of ethyl...
Embodiment 2
[0068] Basic technique is identical with example 1, only changes silane coupling agent to be mercaptosilane (CH 3 O) 3 Si(CH 2 ) 3 SH, to obtain the prepared mercapto-modified adsorbent S-MCMS.
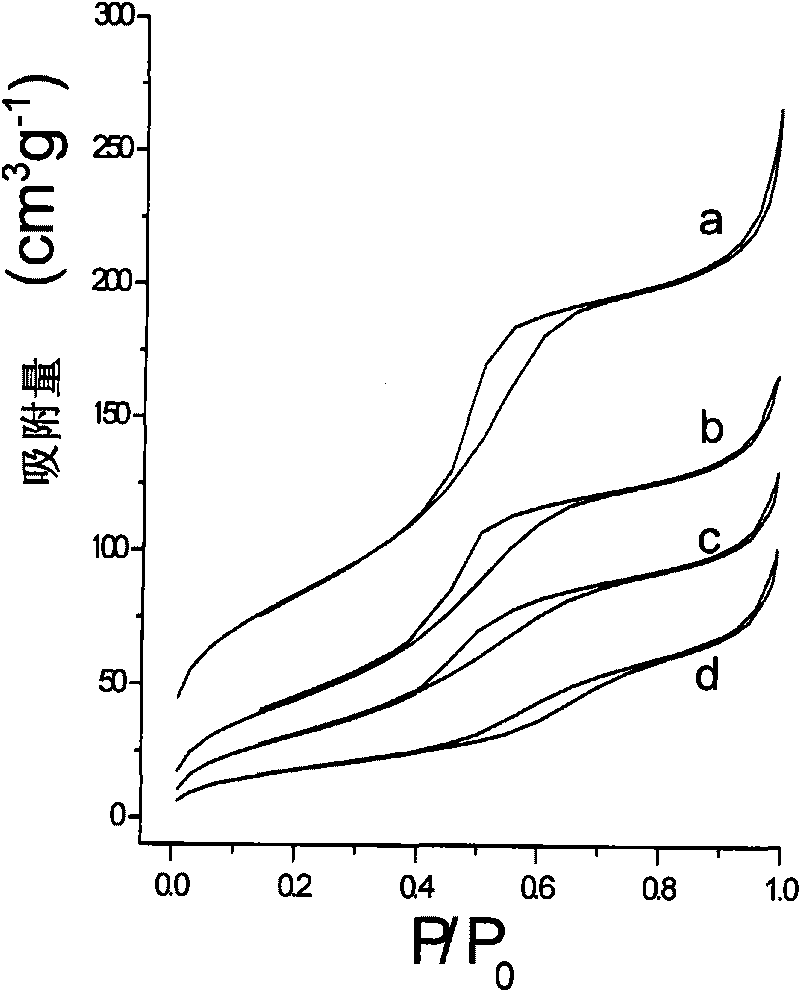
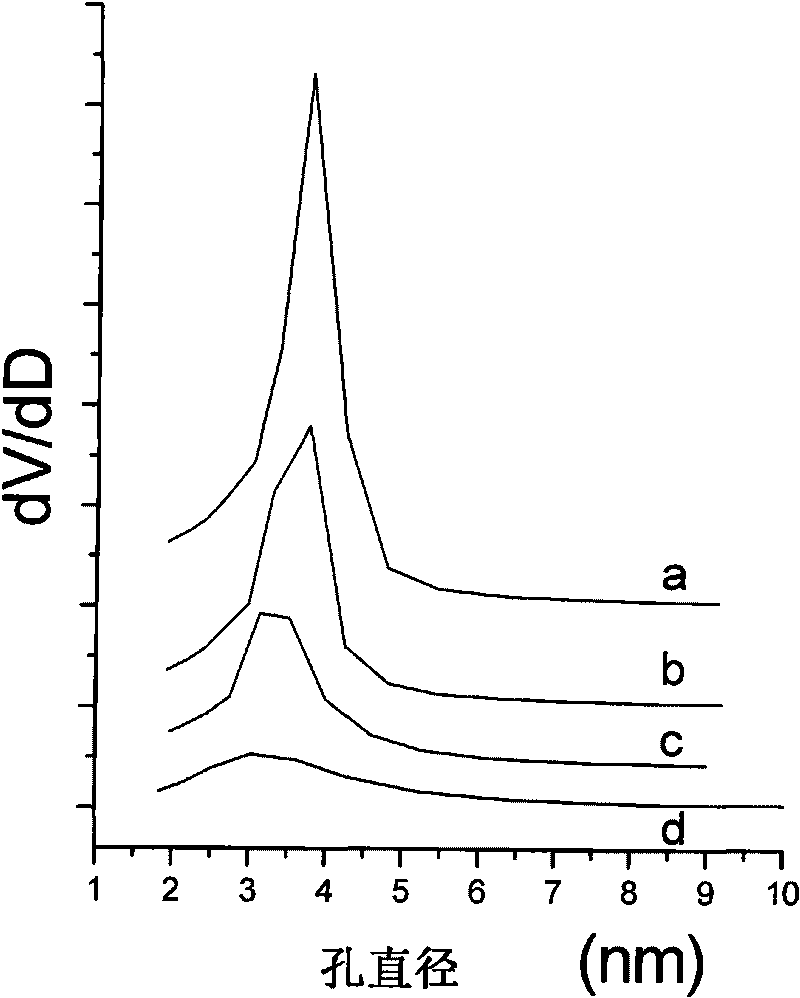
[0069] Its nitrogen adsorption-desorption isotherm shows that after the modification of the mercaptosilane coupling agent, the mesoporous channels of the material still remain good (see figure 2 c and image 3 c). Its transmission electron microscope pictures ( Figure 5 ) showed that after modifying the silane coupling agent, the material maintained a magnetic core-mesoporous shell structure with good dispersion.
[0070] After the adsorbent S-MCMS treats the simulated wastewater, the Hg in the wastewater 2+ , Pb 2+ , Cd 2+ , Zn 2+ , Cu 2+ Concentrations were significantly reduced (Table 1), indicating that the adsorbent has good adsorption properties for common metal ions in wastewater.
Embodiment 3
[0072] Basic technique is identical with example 1, and only changing silane coupling agent is ethylaminosilane (CH 3 O) 3 Si(CH 2 ) 3 NH(CH 2 ) 2 NH 2 , to obtain the prepared amino-modified adsorbent N-MCMS.
[0073] Its nitrogen adsorption-desorption isotherm shows that after the aminosilane coupling agent is modified, the mesoporous pores of the material still remain good (see figure 2 d and image 3 d). Its transmission electron microscope pictures ( Image 6 ) showed that after modifying the silane coupling agent, the material maintained a magnetic core-mesoporous shell structure with good dispersion.
[0074] After the adsorbent N-MCMS treats the simulated wastewater, the Hg in the wastewater 2+ , Pb 2+ , Cd 2+ , Zn 2+ , Cu 2+ Concentrations were significantly reduced (Table 1), indicating that the adsorbent has good adsorption properties for common metal ions in wastewater.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com