Preparation method of nickel phosphide
A technology of nickel phosphide and nickel hypophosphite, applied in phosphide and other directions, can solve the problem that the preparation method of transition metal phosphide is rarely studied.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
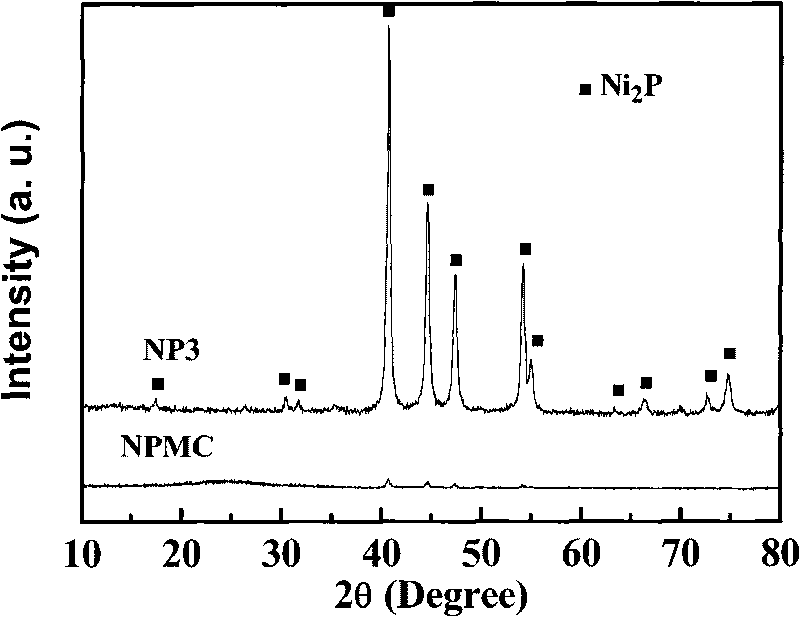
[0024] Press H 2 PO 2 - : Ni 2+ Dissolve nickel hypophosphite and ammonium hypophosphite in deionized water with a ratio of 3, stir, and evaporate to dryness in a 333K water bath. Take 5.0 g of the above precursor, put it in a tube furnace, pass N 2 , heated up to the reaction temperature at a rate of 2K / min, stabilized for 3h, and cooled to room temperature with O 2 Take out after 1% nitrogen passivation for 3 hours. Among them, N 2 The flow rate is 100ml / min, and the final temperature is 473K. The roasted samples were first washed with ammonia until colorless, then washed with deionized water until neutral, and dried. The specific surface area of the prepared sample (named NP3) is 14m 2 / g, XRD spectrum see figure 1 .
Embodiment 2
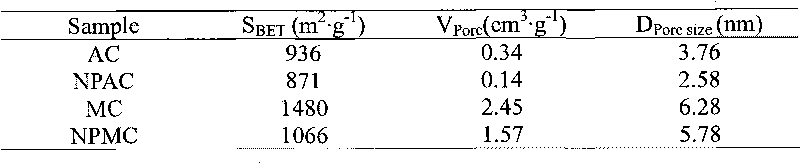
[0026]Dissolve 1.78g of nickel hypophosphite and 0.50g of ammonium hypophosphite in 6.6g of deionized water, add 6.0g of activated carbon (AC, see Table 1 for structural properties, equivalent dipping point 1.1ml / g), stir, and dry in the shade. Get impregnated sample 5.0g and roast, wash and dry in 473K nitrogen, method is the same as embodiment 1. The prepared catalyst was named NPAC. The structural properties of the supports and supported catalysts are listed in Table 1.
[0027] Table 1 Structural properties of catalysts and supports.
[0028]
Embodiment 3
[0030] Dissolve 5.94g of nickel hypophosphite and 1.66g of ammonium hypophosphite in 14.0g of deionized water, add 5.0g of mesoporous carbon (see Table 1 for the structural properties, and the equivalent immersion point is 2.8ml / g), stir, and dry in the shade. Get impregnated sample 5.0g and roast, wash and dry in 473K nitrogen, method is the same as embodiment 1. The prepared catalyst was named NPMC. The structural properties of the carrier and the loaded catalyst are shown in Table 1, and the XRD spectrum is shown in figure 1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com