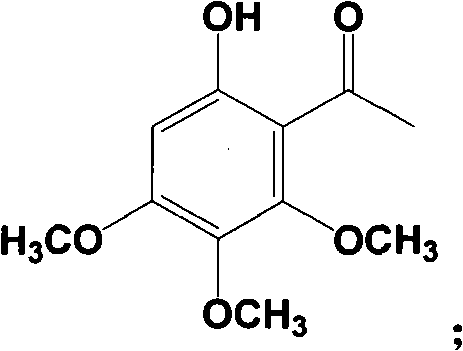
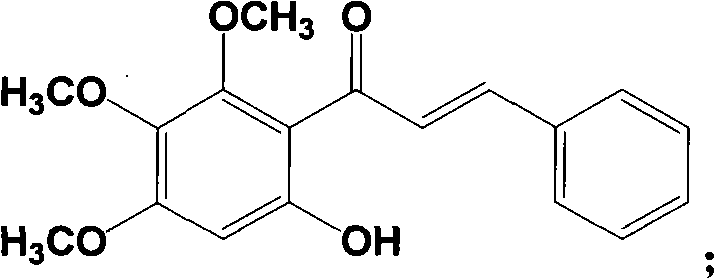
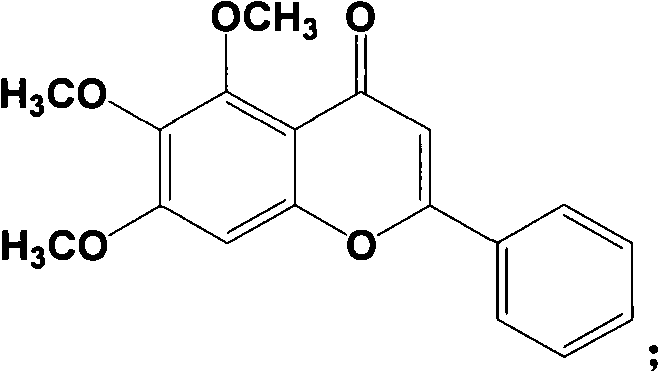
Method for preparing baicalein
A technology for baicalein and a compound, which is applied in the field of preparing baicalein, can solve the problems of difficult preservation of cinnamoyl chloride, complicated synthesis steps, etc., and achieves the effects of simple separation and purification of products, low price and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Step 1: Preparation of compound II
[0035] Weigh 18.4g (0.1mol) of 3,4,5-trimethoxyphenol into a reaction vessel, add about 30ml of acetic anhydride, slowly add 10ml of boron trifluoride ether solution under stirring at room temperature, and then slowly heat to 60°C for reaction 3 hours (TLC checks that the starting material has reacted completely). The above reaction solution was slightly cooled naturally, added with 100 ml of ethyl acetate and stirred, then placed in the refrigerator overnight, and filtered to obtain the precipitated yellow solid. Add 100ml of water and 10ml of ethanolamine to the yellow solid, stir well for 1-2 hours, and then extract the product twice with ethyl acetate, 80ml each time. The extracts were combined, washed once with water, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain compound II as a light yellow oil, which could solidify after refrigeration, and weighed to obtain com...
Embodiment 2
[0043] Step 1: Preparation of compound II
[0044] Weigh 18.4g (0.1mol) of 3,4,5-trimethoxyphenol into a reaction vessel, add about 30ml of acetic acid, slowly add 50g of polyphosphoric acid under stirring at room temperature, then slowly heat to 80°C for 1 hour (TLC Check that the raw material has reacted completely).
[0045] Under stirring, the above reaction solution was poured into 150 ml of ice-cold water while hot, and then the product was extracted twice with ethyl acetate, 100 ml each time. The extracts were combined, washed once with water, dried over anhydrous sodium sulfate, filtered, and the solvent was distilled off under reduced pressure to obtain compound II as a light yellow oil, which could solidify after being refrigerated, dried and weighed, yield: 85%. 1 HNMR (CDCl 3 ):Cit.
[0046] Step 2: Preparation of compound III
[0047] Weigh 22.4g (0.1mol) of compound II and 13g (0.12mol) of benzaldehyde, put them in a 250ml round bottom flask, add 100ml of met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com