Method for synthesizing 4-vinyl-2(5H)-furanone
A synthetic method and vinyl technology, applied in the direction of organic chemistry, can solve the problems of expensive price, expensive reagents, and inability to scale up production, and achieve the effect of solving mass production problems, simple and convenient separation and purification, and low toxicity of reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
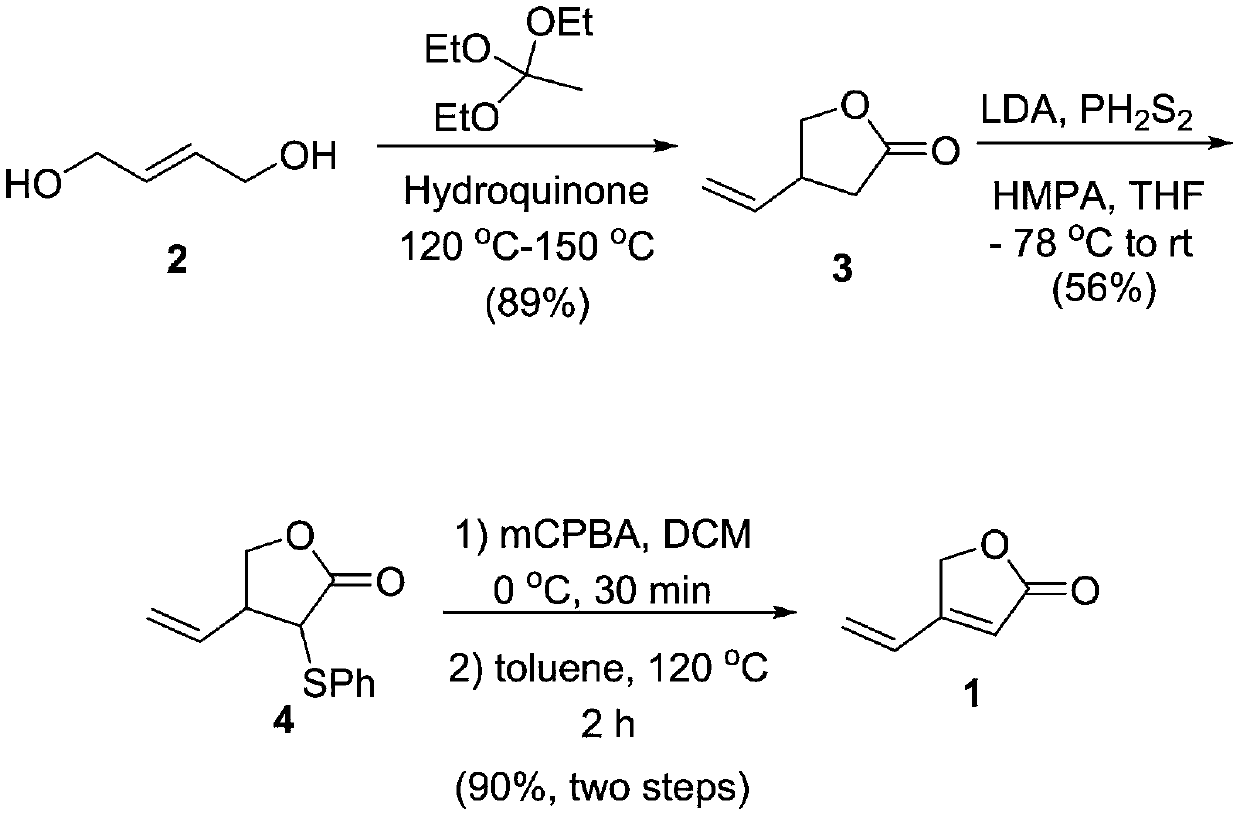
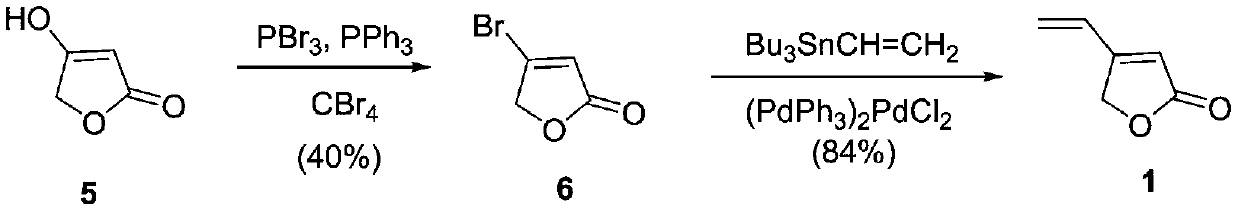
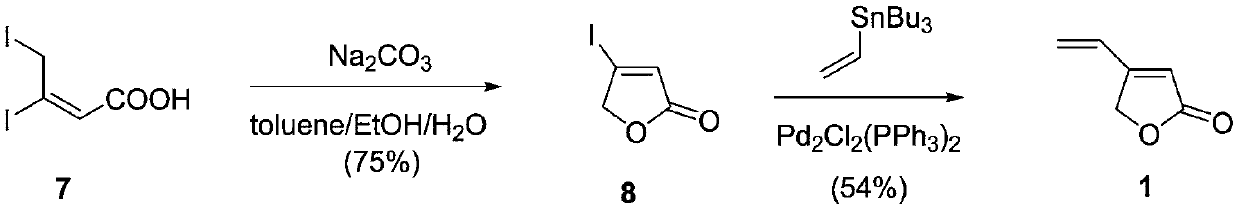
[0024] The synthetic method of 4-vinyl-2(5H)-furanone
[0025]
[0026] (1) Add 250mL of methanol to a 500mL three-necked flask, add 5g of sodium metal at 0°C, and slowly add 24mL of thiophenol (PhSH) dropwise after the Na is completely dissolved, stir at 0°C for 20 minutes, and then add α-bromo - 29g of γ-butyrolactone (compound 9), stirred at room temperature for 2 hours, quenched the reaction by adding water, then extracted with acetic acid, the extract was dried and concentrated, and the concentrated product was separated by silica gel column chromatography to obtain α-phenylthio-γ- Butyrolactone (compound 10) 26g, the reaction yield is 77%.
[0027] The NMR identification results of α-phenylthio-γ-butyrolactone are: 1 H NMR (CDCl 3 ,300MHz)δ2.28 (ddd,J=6.2,10.4,15.7Hz,1H),2.63-2.72(m,1H),3.84-3.88(m,1H),4.19-4.27(m,2H),7.34- 7.35 (m, 3H), 7.55-7.57 (m, 2H).
[0028] (2) 9.3 g of α-phenylthio-γ-butyrolactone (compound 10) and 9.8 g of m-chloroperoxybenzoic acid (m-C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com