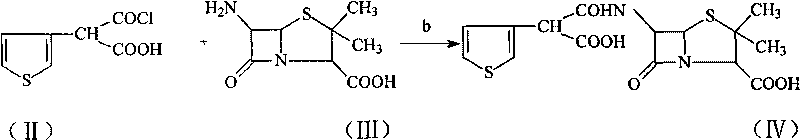
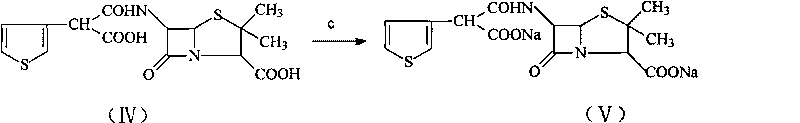
Method for preparing broad-spectrum penicillin antibiotic ticarcillin sodium
A technology of ticarcillin sodium and ticarcillin disodium salt, which is applied in the new synthesis field of ticarcillin sodium salt, can solve the problems of complex salt-forming process, low yield and purity, etc., achieves a simple reaction route and reduces production The effect of improving cost and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
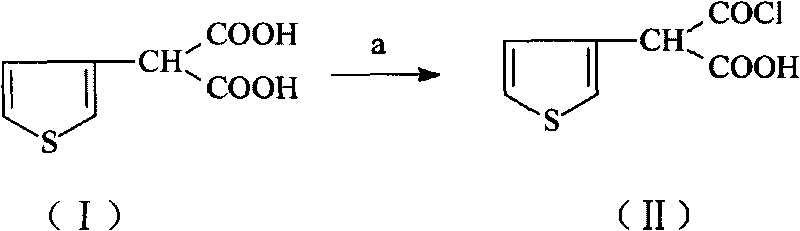
[0039] Embodiment 13-thiophene malonic acid carries out chlorination reaction and prepares compound (II)
[0040] 9.3 grams of 3-thiophene malonate, 60ml of isopropyl ether, 0.2ml of DMF, 3.8ml of SOCl 2Add it to the round-necked bottle in turn, stir and heat up, control the temperature at 54°C to 56°C and reflux for 2 hours. After the reaction is complete, transfer the reaction solution to a rotary evaporator and distill to dryness at 30°C to obtain 9.45g of compound (II). Add 100ml of dichloromethane was used to obtain solution A for the next reaction with a yield of 92.5%.
Embodiment 23
[0041] Embodiment 23-thiophene malonic acid carries out chlorination reaction and prepares compound (II)
[0042] 12 grams of 3-thiophene malonate, 80ml of tetrahydrofuran, 0.5ml of DMF, 4ml of SOCl 2 Add it to the round-necked bottle in turn, stir and heat up, and control the temperature at 65°C to 68°C to reflux for 2.5 hours. After the reaction is complete, transfer the reaction solution to a rotary evaporator, and distill it to dryness under reduced pressure at 30°C to obtain 12.26g of compound (II). 100ml of dichloromethane was used to obtain solution A for the next reaction with a yield of 93%.
Embodiment 33
[0043] Embodiment 33-thiophene malonic acid carries out chlorination reaction and prepares compound (II)
[0044] Add 11 grams of 3-thiophenemalonic acid, 75ml of isopropyl ether, 0.6ml of triethylamine, and 3.9ml of phosphorus oxychloride into the round bottle in turn, stir and heat up, control the temperature at 54°C to 57°C and reflux for 2.5 hours, and the reaction is complete , the reaction solution was transferred to a rotary evaporator, and distilled to dryness under reduced pressure at 30°C to obtain 10.88g of compound (II), and 100ml of dichloromethane was added to obtain solution A for the next reaction, with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com