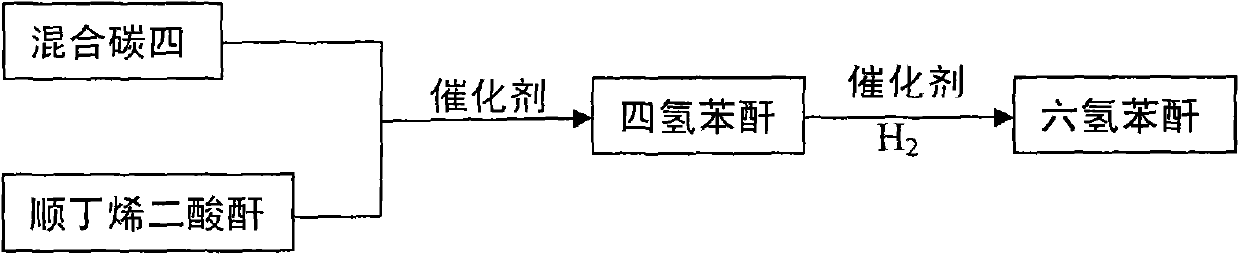
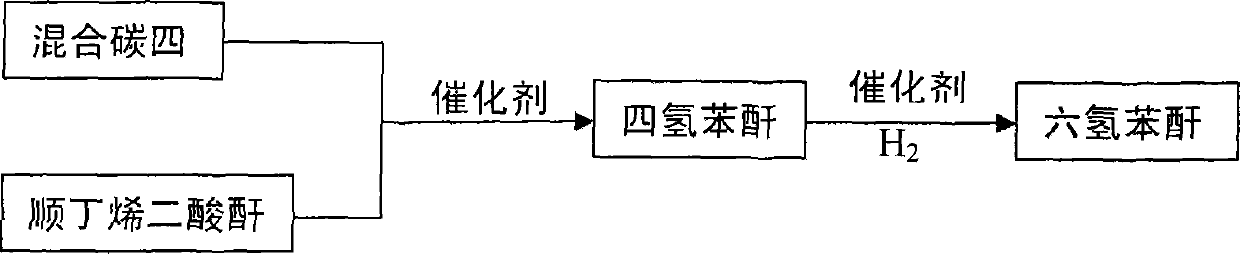
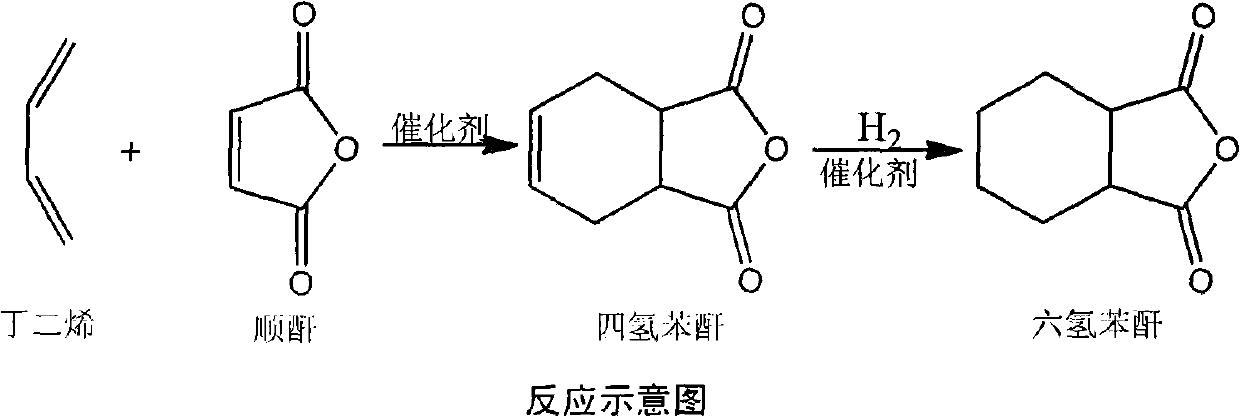
Method for producing hexahydrophthalic anhydride by using C4 mixture
A technology of mixing carbon tetrahydrophthalic anhydride and hexahydrophthalic anhydride, which is applied in organic chemistry and other fields, can solve problems such as high production cost, catalyst poisoning, and influence on reaction conversion rate and selectivity, and achieve low production cost, reduced production cost and energy consumption, The effect of inhibiting the occurrence of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] At 80 ℃, 100 grams of maleic anhydride, 0.009 grams of p-hydroxyanisole and 0.001 grams of [Cu 2 (PPh 3 ) 6 (μ-OOCH))(C)O 4 ), gradually add mixed carbon four (its main component is butadiene), generate tetrahydrophthalic anhydride through Diels-Alder reaction, and add Pd / BaSO to the generated tetrahydrophthalic anhydride after GC detection 4 Catalyst 3.0 g and [Cu 4 (C 7 H 4 NO 4 ) 2 (dppm) 4 ](NO 3 ) 2 0.03 g. Catalytic hydrogenation was carried out in an autoclave with a temperature of 140°C and a hydrogen pressure of 4.0 MPa for 3 hours. After cooling, the catalyst was filtered and distilled under reduced pressure to obtain 139.9 g of colorless liquid hexahydrophthalic anhydride. The yield was 90% and the melting point was 36°C. .
[0017] 1 HNMR(300MHz, CDCl 3 ), δ / ppm: 3.12-3.15 (m, 2H; CH), 1.85-1.91 (m, 4H; CH 2 ), 1.50-1.53(m, 4H; CH 2 );
[0018] 13 CNMR(300MHz, CDCl 3 ), 8 / ppm: 172.8, 40.4, 23.7, 21.9;
[0019] IR(KBr), v / cm -1 : 2948, 2862, 1867, 1787, 1694, 1257, ...
Embodiment 2
[0022] At 150°C, 100 grams of maleic anhydride, 0.08 grams of p-hydroxyanisole and 0.02 grams of [Cu 2 (PPh 3 ) 6 (μ-OOCH))(C1O 4 ), gradually add mixed carbon four (its main component is butadiene), generate tetrahydrophthalic anhydride through Diels-Alder reaction, and add Pd / BaCO to the generated tetrahydrophthalic anhydride after the reaction is detected by GC 3 Catalyst 3.2 g and [Cu 4 (C 7 H 4 NO 4 ) 2 (dppm) 4 ](NO 3 ) 2 0.04 g. Catalytic hydrogenation was carried out in an autoclave with a temperature of 120°C and a hydrogen pressure of 2.0 MPa for 5 hours. After cooling, the catalyst was filtered and distilled under reduced pressure to obtain 143.0 g of colorless liquid hexahydrophthalic anhydride. The yield was 93% and the melting point was 36°C. .
Embodiment 3
[0024] At 120°C, 100 grams of maleic anhydride, 0.042 grams of p-hydroxyanisole and 0.008 grams of [Cu 2 (PPh 3 ) 6 (μ-OOCH))(ClO 4 ), gradually add the mixed carbon four (its main component is butadiene), and generate tetrahydrophthalic anhydride through Diel-Alder reaction. After the reaction is detected by GC, add Pd / BaCO to the generated tetrahydrophthalic anhydride 3 Catalyst 3.0 g and [Cu 4 (C 7 H 4 NO 4 ) 2 (dppm) 4 ](NO 3 ) 2 0.04 g. Catalytic hydrogenation was carried out in an autoclave at a temperature of 100°C and a hydrogen pressure of 1.0 MPa for 6 hours. After cooling, the catalyst was filtered and distilled under reduced pressure to obtain 144.6 g of colorless liquid hexahydrophthalic anhydride. The yield was 92% and the melting point was 35°C. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com