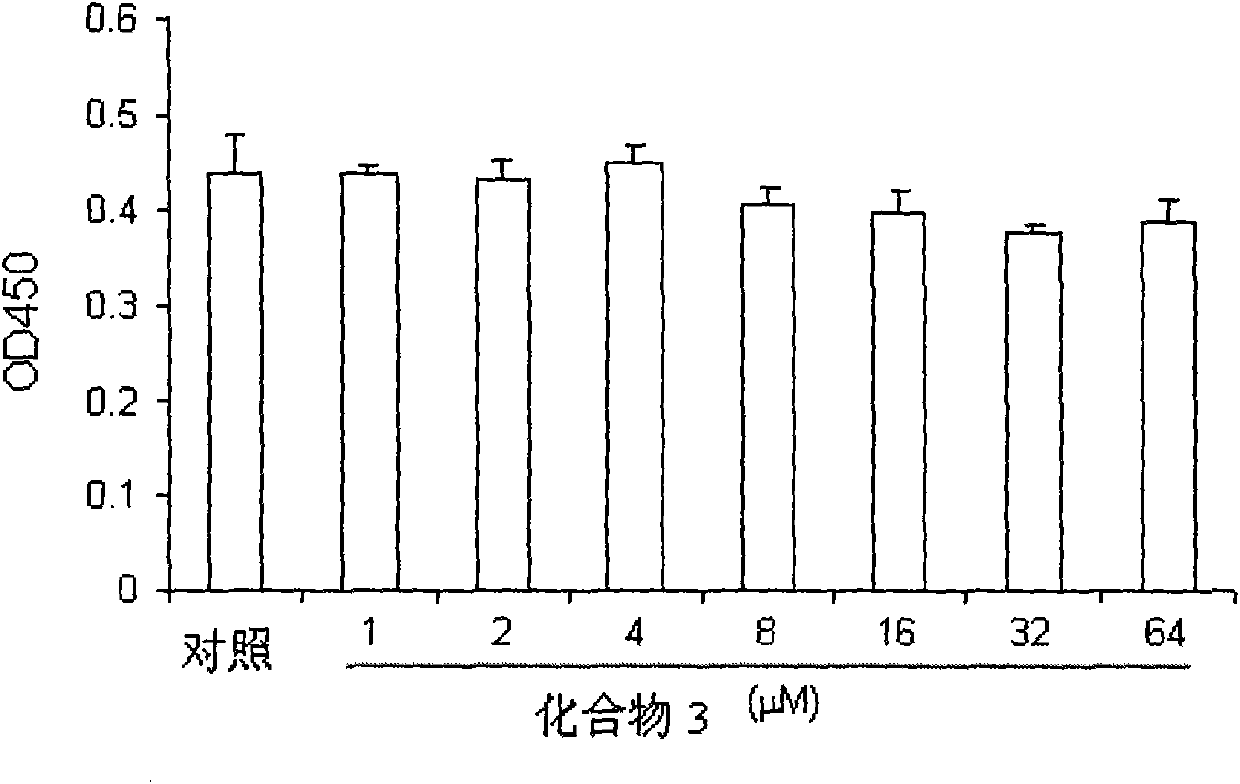
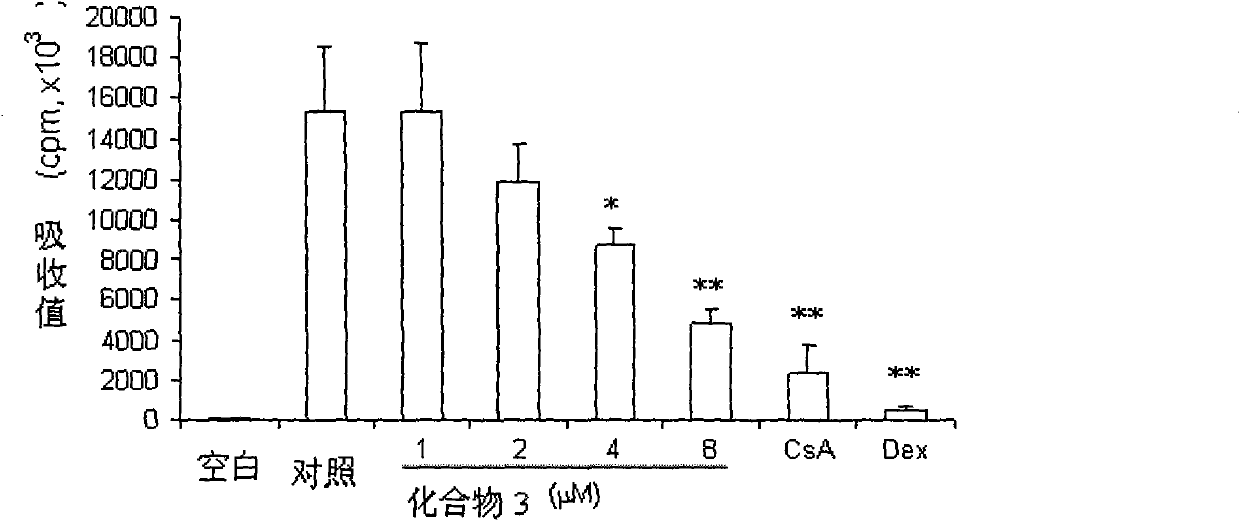
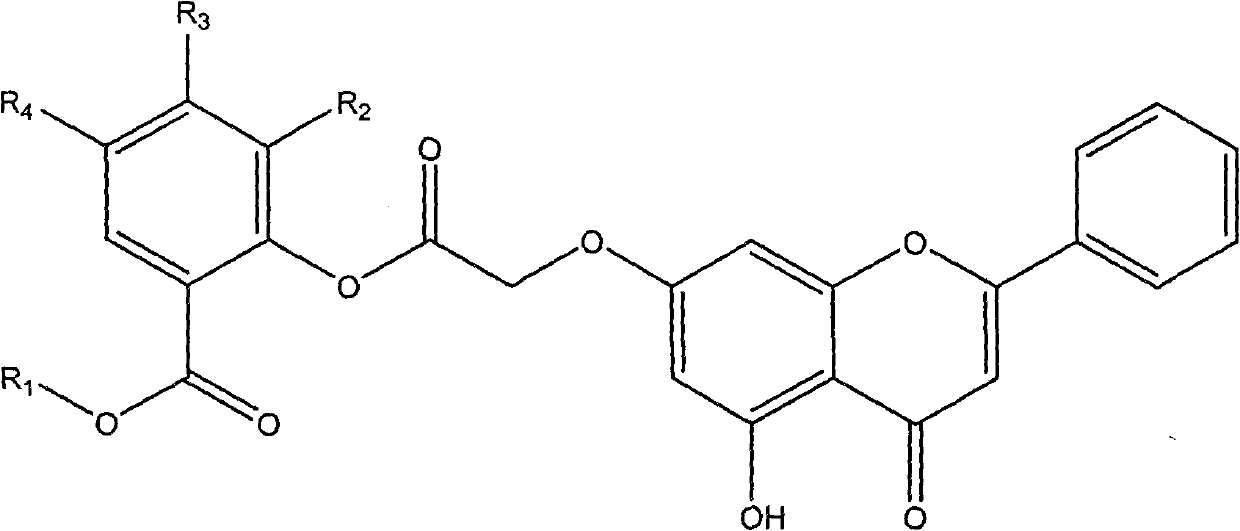
Chrysin and substituted salicylate composites, manufacturing method thereof and use thereof
A technology of salicylate and chrysin, which is applied to a class of chrysin and substituted salicylate complexes and the fields of their preparation and use, and can solve problems such as easy side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of ethyl 2-(5-hydroxy-2-phenyl-4H-benzopyrone-7-oxo)acetate
[0021]
[0022] Chrysin (2.54g, 10mmol) and anhydrous potassium carbonate (2.76g, 20mmol) were sequentially added to anhydrous acetone, then ethyl bromoacetate (0.3ml, 15mmol) was added dropwise, and heated to reflux at 60°C for 12h. After complete cooling, filter under vacuum, discard the solids to obtain the filtrate, wash with water (100ml) and then wash with sodium hydroxide solution (10%, 100ml), and finally wash with water (100ml) again, and after drying, obtain the target compound. Pale yellow solid, yield 86%, mp: 243-245°C, 1 H NMR (300MHz, d 6 -DMSO): 1.25(t, J=6.9Hz, 3H); 4.19(m, 2H); 4.96(s, 2H); 6.43(m, 1H); 6.85(m, 1H), 7.01(s, 1H) , 7.60 (m, 3H), 8.10 (m, 2H), 12.81 (s, 1H). MS (ESI): 340.1 (C 19 h 16 o 6 ,[M+H] + ).Anal.Calcd for C 19 h 16 o 6 : C, 67.05; H, 4.74%; Found: C, 67.08; H, 4.72%.
Embodiment 2
[0023] Example 2: Preparation of 2-(5-hydroxyl-2-phenyl-4H-benzopyrone-7-oxo)acetic acid
[0024]
[0025] The product 2-(5-hydroxy-2-phenyl-4H-benzopyrone-7-oxo)ethyl acetate (3.4g, 10mmol) obtained in Example 1 was dissolved in dimethyl sulfoxide (DMSO) After completely dissolving in the solution, add 40ml of 5% sodium carbonate aqueous solution, and heat to reflux at 90°C for 8h. After the reactants were completely reacted, 50ml of 1M hydrochloric acid solution was added, stirred continuously, and allowed to stand for 4h, and a yellow solid was precipitated. After washing with water (100ml) twice, and drying, the title compound was obtained as a yellow solid. Yield 82%, mp: 315-316°C; 1 H NMR (500MHz, d 6 -DMSO): 2.51 (s, 3H); 4.85 (s, 2H); 6.41 (m, 1H), 6.82 (m, 1H); 7.04 (s, 1H); 7.61 (m, 3H), 8.11 (m, 2H), 12.80(s, 1H), 13.20(s, 1H). MS (ESI): 312.1 (C 17 h 12 o 6 ,[M+H] + ).Anal.Calcd for C 17 h 12 o 6 : C, 65.39; H, 3.87%; Found: C, 65.37; H, 3.85%.
Embodiment 3
[0026] Example 3: Preparation of 5-hydroxy-2-[2-(5-hydroxy-2-phenyl-4H-benzopyrone-7-oxo)acetoxy]-benzoic acid methyl ester (compound 1) .
[0027]
[0028]The product 2-(5-hydroxyl-2-phenyl-4H-benzopyrone-7-oxo)acetic acid (3.12g, 10mmol) obtained in Example 2 was dissolved in 30ml N,N-dimethylformaldehyde amide (DMF), then added 5-hydroxymethylsalicylate (1.68g, 10mmol), N,N-dimethylaminopyridine (DMAP) (61mg, 2.03mmol) and N,N-dicyclohexyl carbon Diimine (DCC) (3.08g, 11mmol) was heated to reflux at 95°C for 12h. After the reaction was complete, it was extracted with ethyl acetate, and the organic layer was washed with saturated brine, and then washed with anhydrous Na 2 SO 4 After drying, the solvent is evaporated to dryness under reduced pressure and separated with a silica gel column. The eluent is: ethyl acetate: sherwood oil = 1: 2-1: 5 to obtain the above-mentioned chrysin and 5-hydroxysalicylate complex of the present invention . White powder. The yield was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com