New preparation method of hydrochloride landiolol
A technology of landisolol hydrochloride and propionic acid, which is applied in the fields of cardiovascular system diseases, organic chemistry, and drug combination, and can solve the problems of high reactivity of boron trifluoride ether, which is unfavorable for industrial production, and unfavorable for practical operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Synthesis of N-(2-aminoethyl)morpholine formamide (intermediate II)
[0050] Take 70 g of N-(2-aminoethyl) morpholine formamide oxalate and add it into a 2 L three-necked flask, add 1 L of 95% ethanol, and stir. Ice-water bath, add sodium hydroxide 18.9g to the three-neck flask in batches. After addition, stir for 7-18 hours. Add anhydrous sodium sulfate to dry. Filter and dry the filtrate under reduced pressure below 40°C. The oil was dissolved in 300ml of dichloromethane, and dried by adding anhydrous sodium sulfate. Filter and dry the filtrate under reduced pressure below 40°C. The oil was placed at low temperature to obtain 38.2 g of solid. Yield = 68.7%
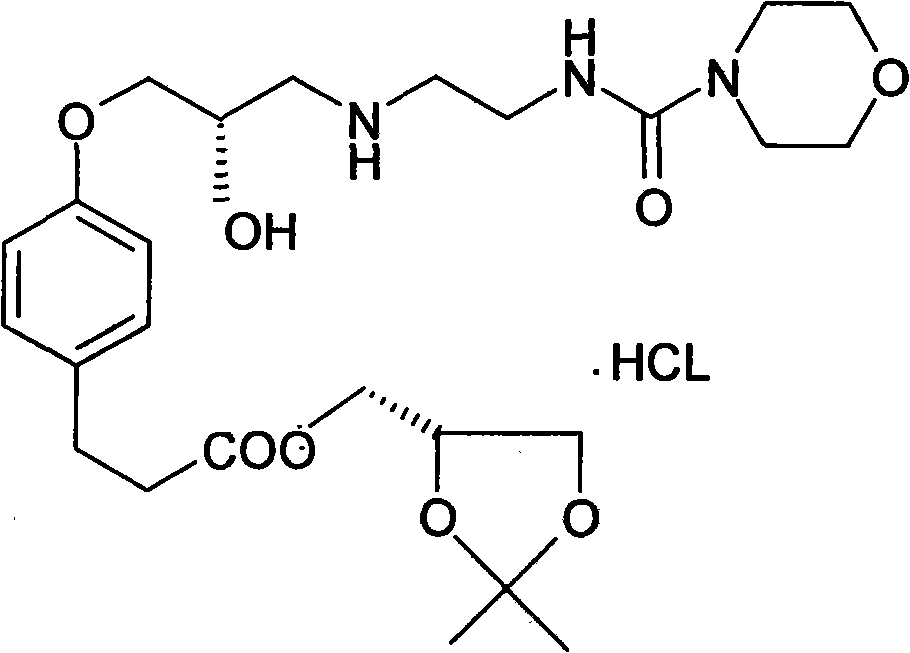
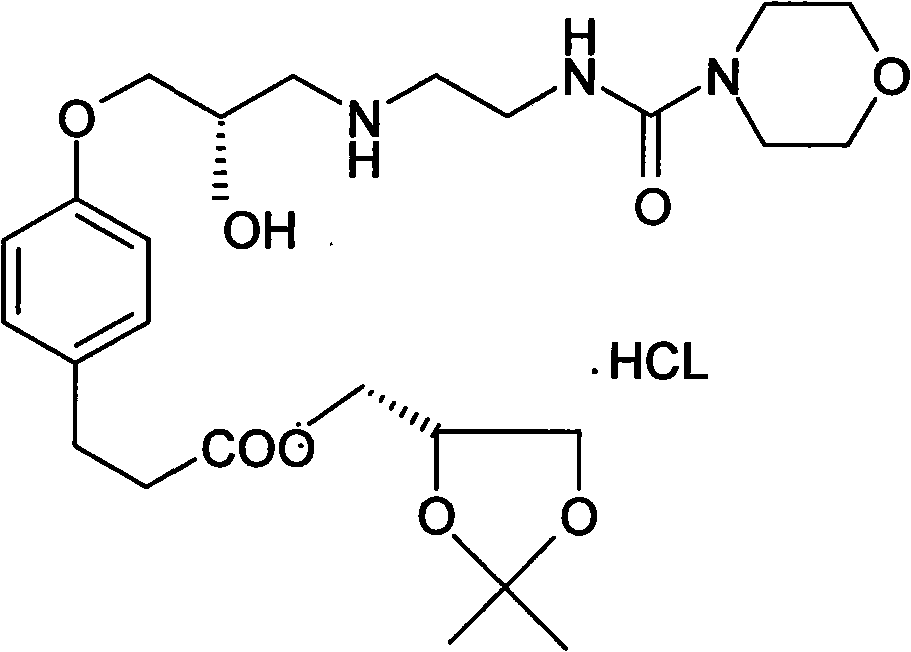
[0051] (2) 3-{4-[2S-Hydroxy-[3-(2-morpholinecarboxamido)ethyl]-aminopropoxy]-phenyl}propanoic acid (2,2-dimethyl-1 , Synthesis of 3-dioxolane-4S)methyl ester (Intermediate III).
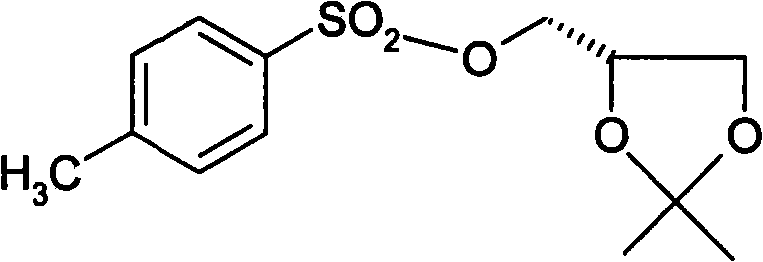
[0052] Take 22.4g of raw material II and 80ml of dimethyl sulfoxide into a 250L three-neck flask respectively, stir, and ad...
Embodiment 2
[0061] (1) Synthesis of N-(2-aminoethyl)morpholine formamide (intermediate II)
[0062] Add 70 g of N-(2-aminoethyl) morpholine formamide oxalate into a 2 L three-necked flask, add 1 L of methanol, and stir. Ice-water bath, add sodium hydroxide 18.9g to the three-neck flask in batches. After addition, stir for 7-8 hours. Add anhydrous sodium sulfate to dry.
[0063] Filter and dry the filtrate under reduced pressure below 40°C. The oil was dissolved in 300ml of dichloromethane, and dried by adding anhydrous sodium sulfate. Filter and dry the filtrate under reduced pressure below 40°C. The oil was placed at low temperature to obtain 37.8 g of solid. Yield = 68.0%
[0064] (2) 3-{4-[2S-Hydroxy-[3-(2-morpholinecarboxamido)ethyl]-aminopropoxy]-phenyl}propanoic acid (2,2-dimethyl-1 , Synthesis of 3-dioxolane-4S)methyl ester (Intermediate III).
[0065] Take 22.4g of raw material II and 80ml of dimethyl sulfoxide into a 250L three-neck flask respectively, stir, and add 3.5g ...
Embodiment 3
[0074] (1) Synthesis of N-(2-aminoethyl)morpholine formamide (intermediate II)
[0075] Take 70 g of N-(2-aminoethyl) morpholine formamide oxalate and add it to a 2 L three-necked flask, add 1 L of isopropanol, and stir. Ice-water bath, add sodium hydroxide 18.9g to the three-neck flask in batches. After addition, stir for 7-8 hours. Add anhydrous sodium sulfate to dry. Filter and dry the filtrate under reduced pressure below 40°C. The oil was dissolved in 300ml of dichloromethane, and dried by adding anhydrous sodium sulfate.
[0076] Filter and dry the filtrate under reduced pressure below 40°C. The oil was placed at low temperature to obtain 37.5 g of solid. Yield = 67.5%
[0077] (2) 3-{4-[2S-Hydroxy-[3-(2-morpholinecarboxamido)ethyl]-aminopropoxy]-phenyl}propanoic acid (2,2-dimethyl-1 , Synthesis of 3-dioxolane-4S)methyl ester (Intermediate III).
[0078] Take 22.4g of raw material II and 80ml of dimethyl sulfoxide into a 250L three-neck flask respectively, stir, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com