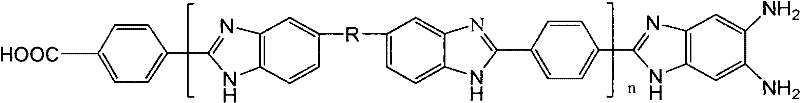
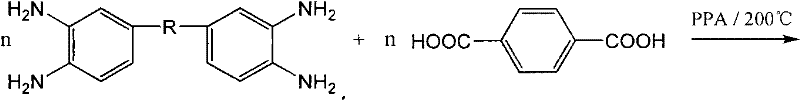Method for protecting amino end groups in polybenzimidazole high-molecular material
A technology of polybenzimidazole and polymer materials, which is applied in the field of protection of terminal amino groups, can solve the problems affecting the stability and service life of materials, and achieve the effect of high yield and easy reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The reaction equation is as follows:
[0054]
[0055] In the formula: (1) is polybenzimidazole; (2) is urea; (3) is PBI whose terminal amino group is protected (that is, a polymer that generates terminal benzimidazolone)
[0056] In a 250mL four-necked flask equipped with a stirrer, a reflux condenser, a thermometer, and a dropping funnel, polybenzimidazole (15g) to be protected, urea (18~28g), 70mL of dimethylacetamide, and 70mL of dimethylacetamide were added successively. heating. Stir under the protection of nitrogen, heat the oil bath to 100 ℃ ~ 108 ℃, add 95% sulfuric acid dropwise, maintain the pH value of the reaction solution between 5 and 6, and react at 100 ℃ ~ 180 ℃. 4.5 g of urea was added every 1 h, and the reaction was continued for 6 h. The reaction mixture was slowly poured into water for spinning, and washed with deionized water for 3 to 5 times. The solid polymer was pulverized and washed with deionized water for 3 times, and the PBI (light yell...
Embodiment 2
[0057] [Example 2] Microwave-assisted solid-phase synthesis
[0058] A microwave-assisted solid-phase synthesis method was used. In a three-necked flask equipped with a condenser and protected by an inert gas, add the polybenzimidazole to be protected, urea, water and concentrated sulfuric acid (the amount added is the same as in Example 1), first feed the protective gas nitrogen for 10 min, and under nitrogen protection Under the microwave intermittent heating reaction, each heating 3mim, intermittent 10min, after the temperature drops, add 5g urea. Such an intermittent reaction is performed 6 to 8 times. The reaction solution was slowly poured into water for spinning. Other processing methods are the same as in Example 1.
Embodiment 3
[0060] With the method of embodiment 1, just replace urea with equimolar substituted urea, thiourea and substituted thiourea to obtain other protection situations in the general formula.
[0061] The polybenzimidazole can be of any structure, but the amount of urea added during the PBI reaction of different structures is different. For example, the amount of urea required by general long-chain PBI is 2 to 4 times the amount of its terminal amino groups. The hyperbranched PBI has a large amount of urea due to its large amount of terminal amino groups. The amount of urea is 2 to 5 times that of its terminal amino group. If the quality of PBI is used as a benchmark, the amount of urea used in hyperbranched PBI is 5 to 10 times that of chain PBI.
[0062] The solvent can be selected from dimethylacetamide, dimethylsulfoxide, N-methylpyrrolidone and the like.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| elongation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com