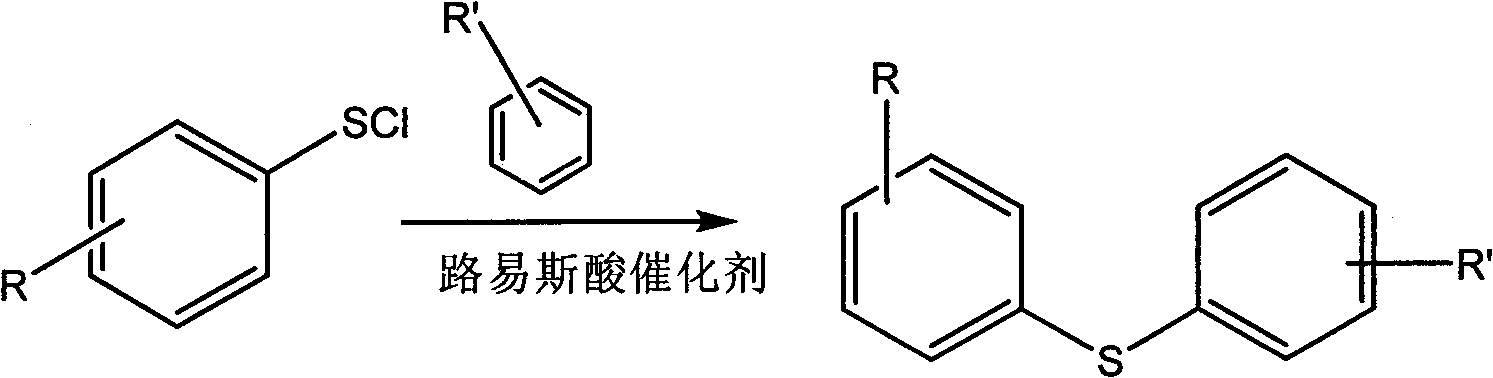
Preparation method for substituted diphenyl sulfide
A technology of diphenyl sulfide and chlorinated alkanes, which is applied in the field of preparation of substituted diphenyl sulfide, can solve the problems of high cost, complex reaction process, complex reaction process, etc., and achieve mild and stable synthesis reaction, high conversion rate of raw materials, Good product selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the synthesis of 4-fluorodiphenyl sulfide
[0016] Add diphenyl disulfide (88g, 0.4mol) and 300mL tetrachloride carbon, turn on the magnetic stirring, and slowly pass in the chlorine gas dried by concentrated sulfuric acid for 3 hours after it is completely dissolved; during this process, keep the internal temperature of the reaction solution not exceeding 20°C with a water bath, and then keep stirring at this temperature for 2 hours to stop the reaction; normal pressure Distill carbon tetrachloride and vacuum distillation to obtain 51g of red benzenesulfenyl chloride with a boiling point of 40-49°C / 2mmHg, with a yield of 88%. Aluminum (16g, 0.12mol), start stirring, and keep the temperature in the bottle in a water bath at 25°C, then add the prepared benzenesulfenyl chloride (11.3g, 0.1mol) dropwise for 1 hour; Continue to stir for 2 hours to complete the reaction; then slowly pour the reaction mixture into a stirred 200g ice-water mixture, separate laye...
Embodiment 2
[0017] Embodiment 2: the synthesis of 4-chlorodiphenyl sulfide
[0018] First synthesize 4-chlorobenzenesulfenyl chloride according to the corresponding steps and methods in Example 1; then add 100mL benzene and 100mL carbon tetrachloride, anhydrous aluminum trichloride (16g, 0.12mol) in a 500mL flask, and start stirring , the water bath kept the temperature in the bottle at 25°C, then added dropwise the prepared 4-chlorobenzenesulfenyl chloride (18g, 0.1mol) for 2 hours; after the dropwise addition, continued to stir for 2 hours at a temperature of 25°C to complete the reaction; then Slowly pour the reaction mixture into a stirred 200g mixture of ice and water, separate layers, and dry over anhydrous magnesium sulfate; finally remove carbon tetrachloride and unreacted benzene by vacuum distillation and collect The distillate obtained 19.5 g of colorless liquid with a yield of 74%, which was 4-chlorodiphenyl sulfide product; 1 HNMR (400M, CDCl 3 , TMS): δ=7.03 (ddt, J=8.8Hz,...
Embodiment 3
[0019] Embodiment 3: the synthesis of 4-chloro-4'-methyl diphenyl sulfide
[0020] First synthesize 4-chlorobenzenesulfenyl chloride according to the corresponding steps and methods in Example 1; then add 100mL toluene and 100mL dichloroethane, anhydrous zinc chloride (16g, 0.12mol) in a 500mL flask, start stirring, The water bath kept the temperature in the bottle at 25°C, and then added dropwise the prepared 4-chlorobenzenesulfenyl chloride (18g, 0.1mol) for 2 hours; after the dropwise addition, continued to stir for 2 hours at a temperature of 25°C to complete the reaction; then put The reaction mixture was slowly poured into a stirred 200g mixture of ice and water, separated into layers, and dried over anhydrous magnesium sulfate; finally, carbon tetrachloride and unreacted toluene were removed by atmospheric distillation, and the fraction with a boiling point of 183-185°C / 266Pa was collected by vacuum distillation Obtained 16.7 g of colorless liquid with a yield of 71%, n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com