Entecavir salt compound, preparation method and medicine application thereof
A technology of entecavir and compound, which is applied in the pharmaceutical field to achieve the effects of good stability, improved water solubility and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Add 10ml of methanol, 1.0g of entecavir and 0.62g of p-toluenesulfonic acid into a 25ml eggplant-shaped bottle, stir, heat to reflux for 4h, cool to room temperature, evaporate part of the solvent under reduced pressure, cool the residue to 0°C, filter with suction, and dry in vacuo , an off-white solid was obtained, which was recrystallized from methanol to obtain 1.2 g of white crystalline entecavir p-toluenesulfonate, with a yield of 74.1%.
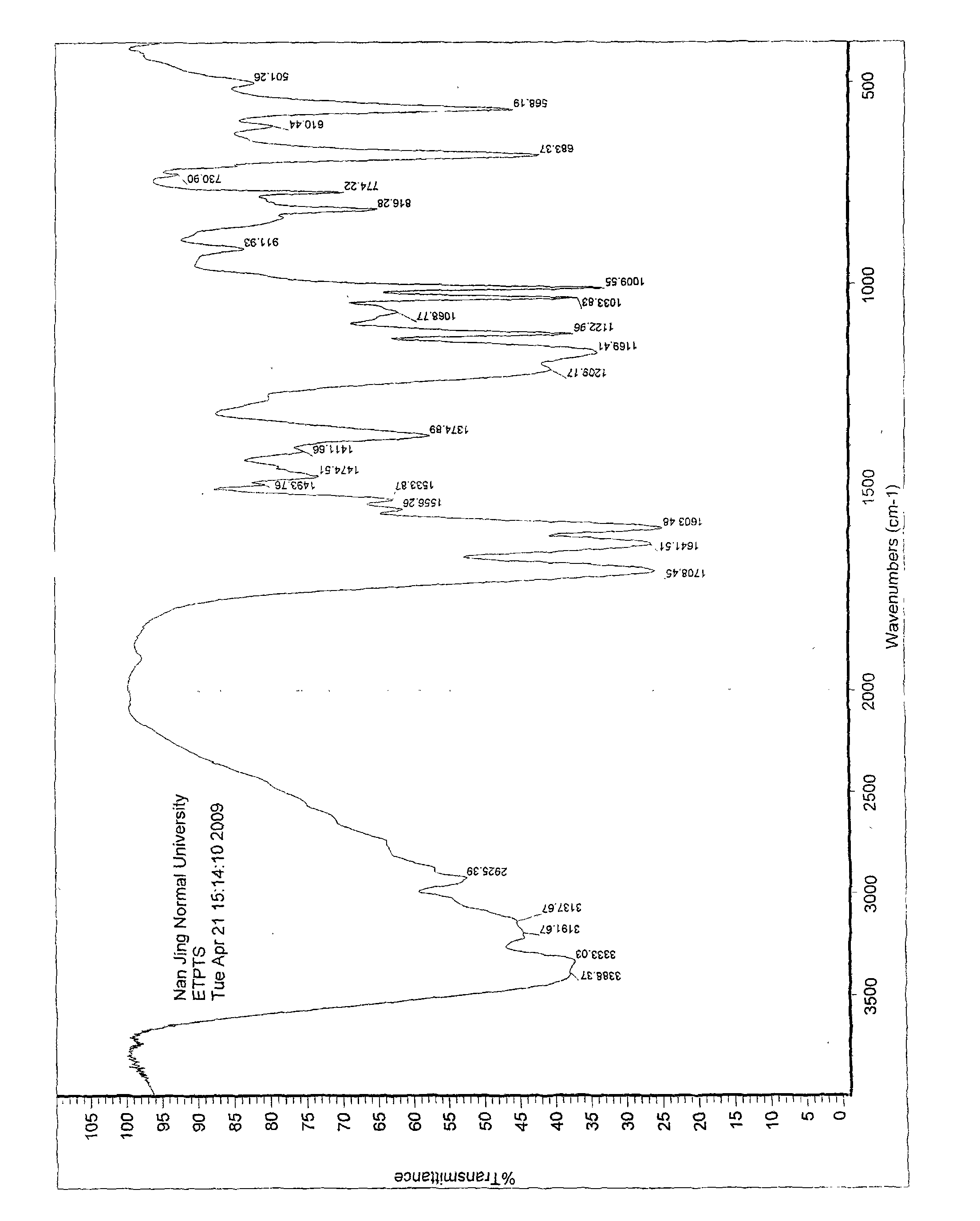
[0056] IR(KBr)cm -1 :
[0057] 3388, 3333, 3191, 3137, 3100~2800, 2925, 1708, 1641, 1603, 1556, 1533, 1474, 1411, 1374, 1169, 1122, 1033, 1009.
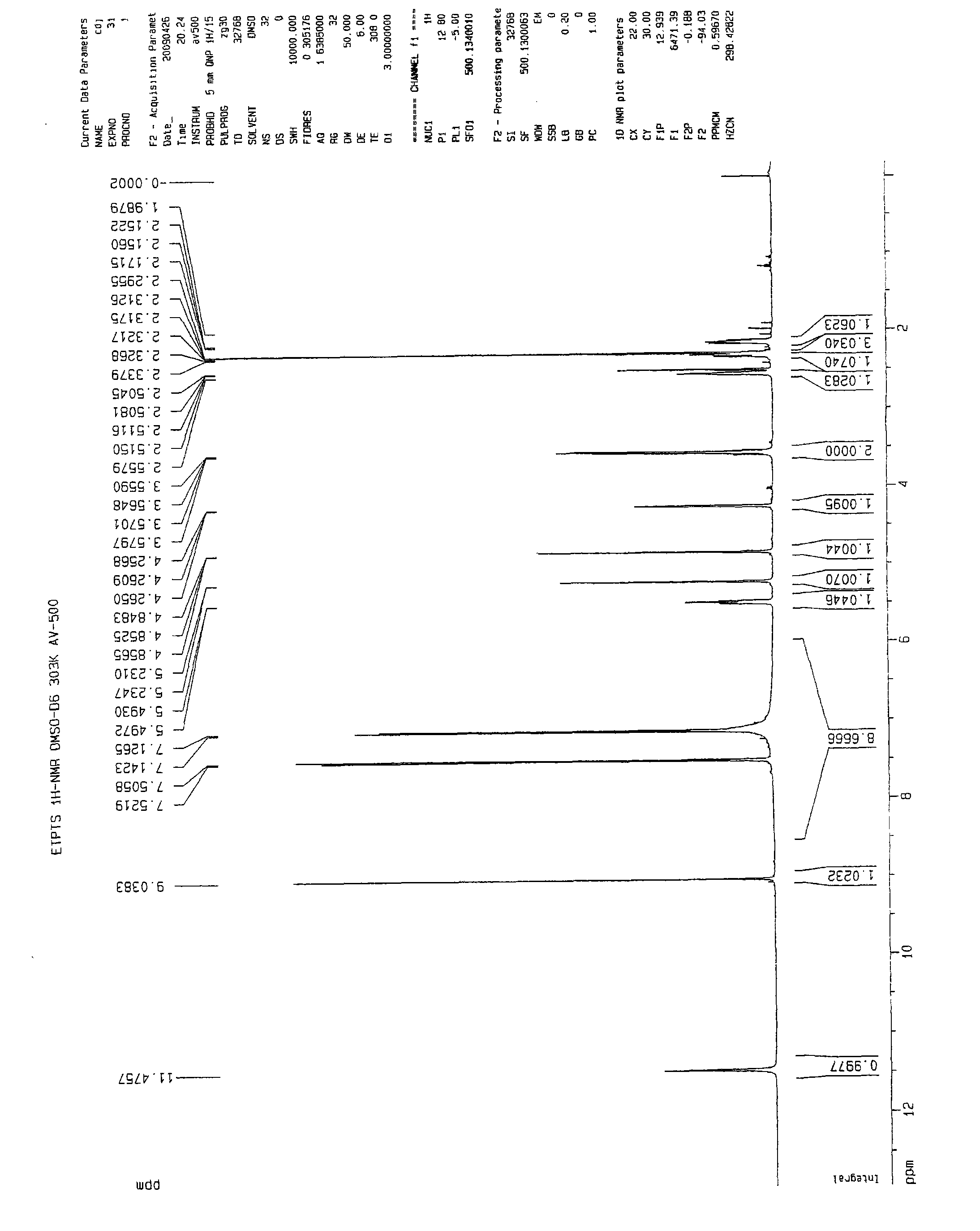
[0058] 1 HNMR (DMSO-d 6 )δ:
[0059] 11.48 (brs, 1H, D 2 Disappeared after O exchange), 9.04 (s, 1H), 7.51 (d, 2H), 7.13 (d, 2H), 7.00~7.80 (brs, 5H, D 2 O disappears after exchange), 5.51~5.48(m, 1H), 5.23(m, 1H), 4.85(m, 1H), 4.26(m, 1H), 3.54~3.60(m, 2H), 2.56(m, 1H ), 2.31~2.35(m, 1H), 2.29(s, 3H), 2.15~2.17(m, 1H).
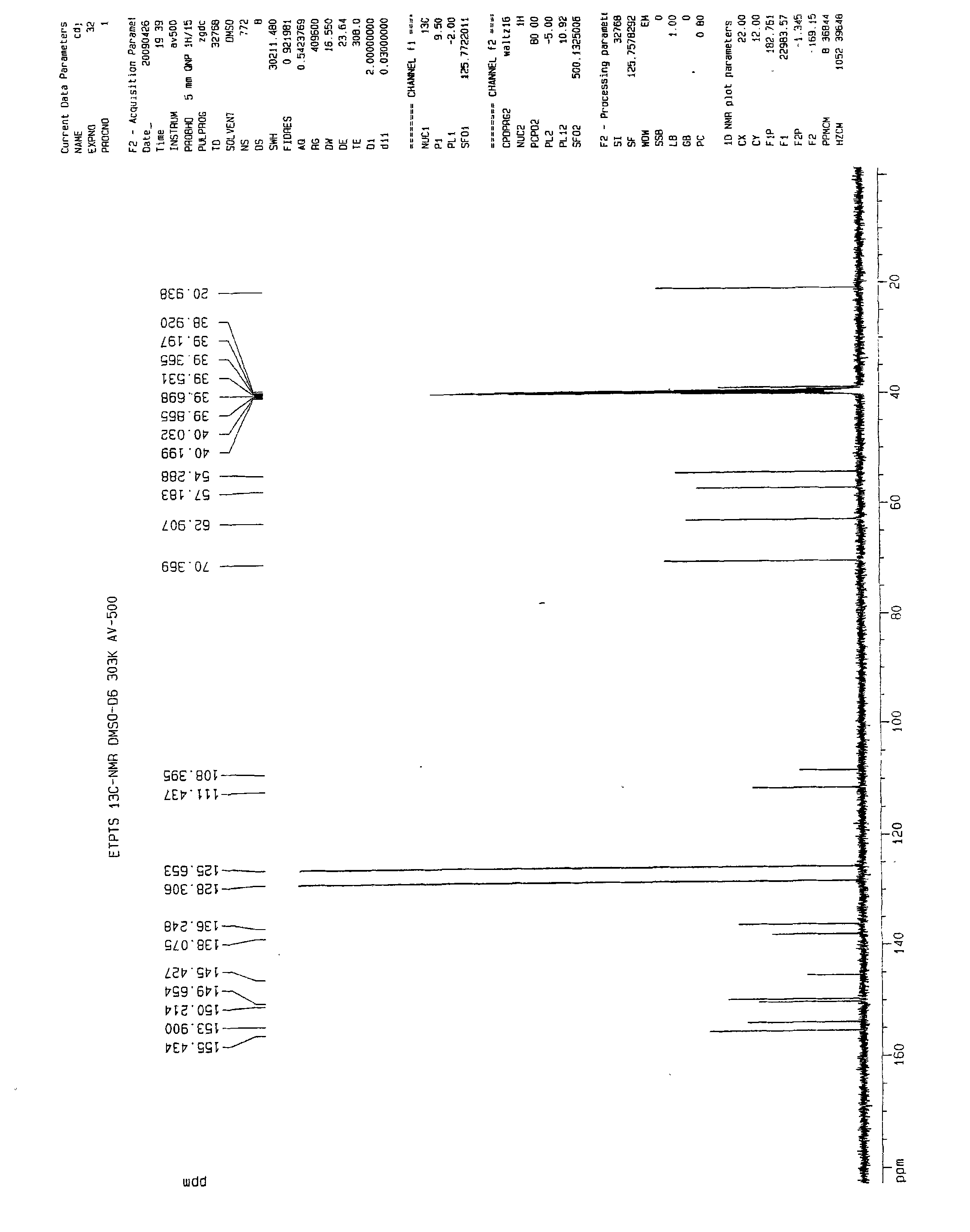
[0060] 13 CNMR (DMSO-d 6 ):
[0061] 155.43, 153.90, ...
Embodiment 2
[0065] Add 35ml of water, 1.0g of entecavir and 0.62g of p-toluenesulfonic acid into a 50ml reaction flask, stir, react at 80°C for 2h, cool to room temperature, evaporate the solvent under reduced pressure, and recrystallize the residue with methanol to obtain white crystals of p-toluenesulfonate Entecavir acid 1.3g, yield 80.2%.
Embodiment 3
[0067] Add 30ml of ethanol, 2.0g of entecavir and 1.25g of p-toluenesulfonic acid into a 50ml eggplant-shaped bottle, stir, heat to reflux for 3h, cool to room temperature, evaporate part of the solvent under reduced pressure, cool the residue to 0°C, filter with suction, and dry in vacuo , an off-white solid was obtained, which was recrystallized from ethanol to obtain 2.0 g of white crystalline entecavir p-toluenesulfonate, with a yield of 61.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com