Synthesis method of Azasetron intermediate
A technology for azasetron and intermediates, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as reduction, incomplete reaction, and unsatisfactory product purity, and achieve high yield and reduction thorough effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Add 20kg of 3-nitro-5-chlorosalicylic acid methyl ester to 100L of industrial glacial acetic acid and stir for 30 minutes, then add 100kg of water and heat up to 30-100°C; add 100-200 mesh industrial reduced iron powder 16kg, After adding in 3 hours, control the temperature at 60-70°C; after adding, react for 5 hours;
[0040] (2) Add 100 kg of glacial acetic acid in batches to the reaction solution of step (1), stir at 70° C. for 30 minutes, filter with diatomaceous earth, and remove iron sludge;
[0041] (3) The filter cake is rinsed with 200L of ethyl acetate, the combined filtrate is stirred for 10 minutes, left to stand for 30 minutes, and separated; and extracted once with 100L of ethyl acetate; the combined ethyl acetate solution is washed 3 times with water to obtain 5-chloro- 3 ethyl acetate solution of aminomethyl salicylate; standby;
[0042](4) Add the ethyl acetate solution of 5-chloro-3 aminosalicylic acid methyl ester of step (3) to saturated NaHCO ...
Embodiment 2
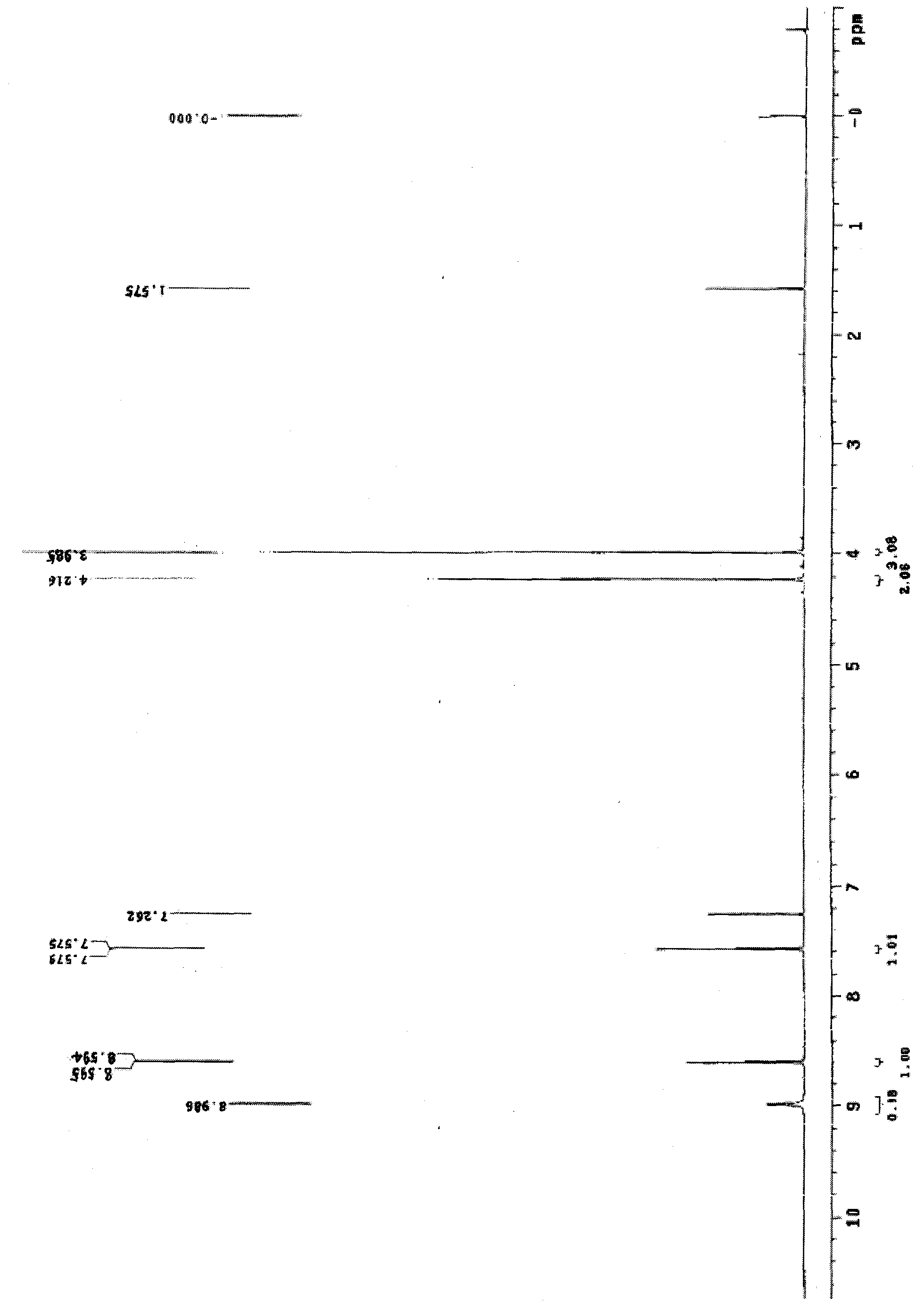
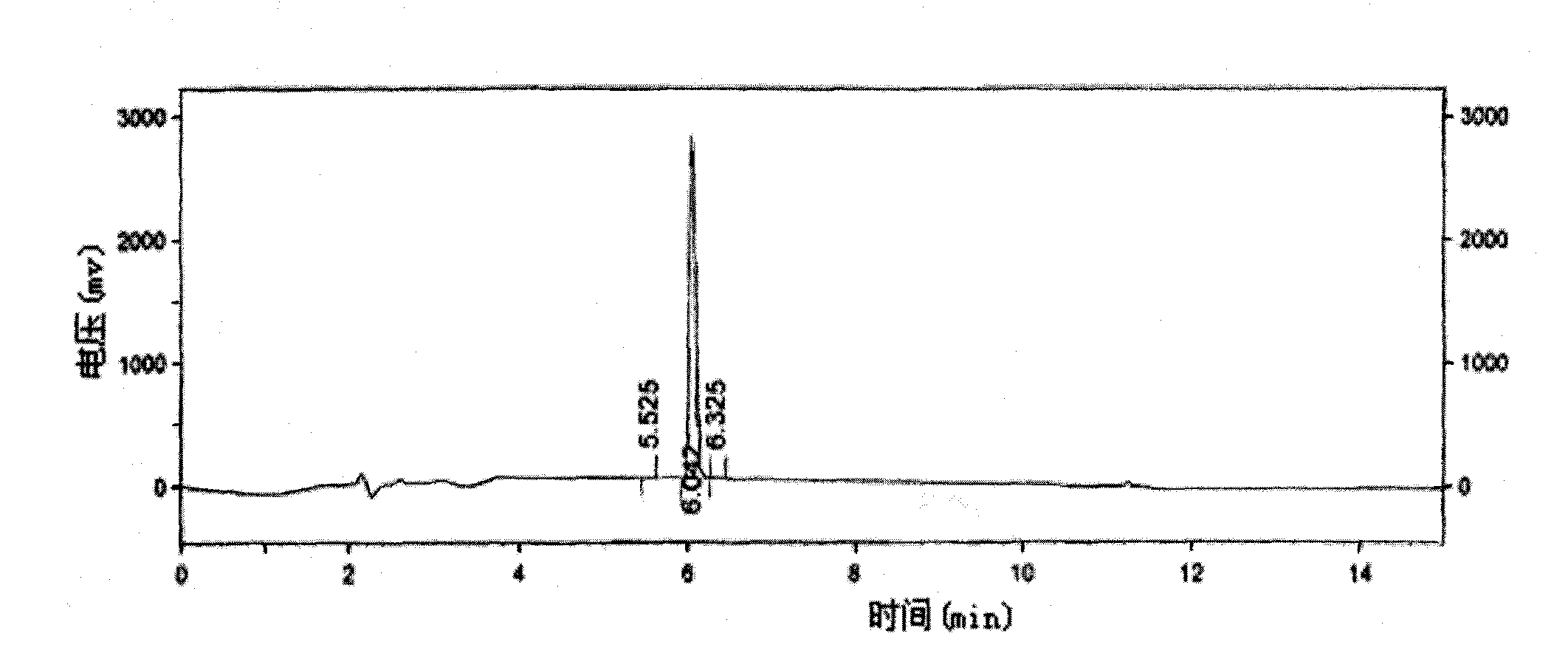
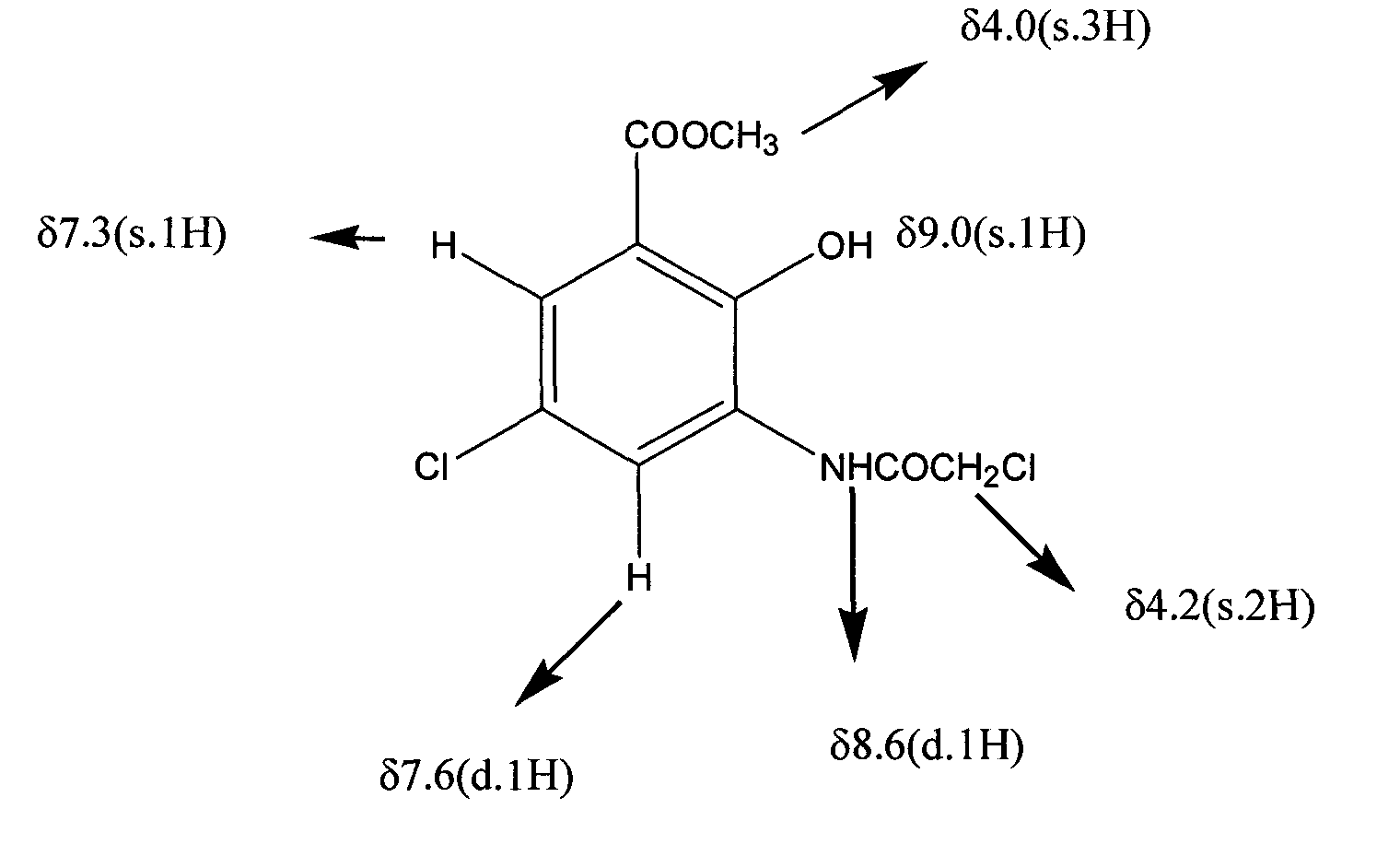
[0046] As described in Example 1, the difference is that the organic solvent of step (3), (5) uses chloroform to obtain 22kg of 3-(2-chloroacetylamino)-5 chloromethyl salicylate, yield 95 %, Mp: 163-164 °C HPLC: 99%.
Embodiment 3
[0048] As described in Example 1, the difference is that the reduced iron powder in step (1) is 15kg, and 22kg of 3-(2-chloroacetylamino)-5-chloromethyl salicylate is obtained, with a yield of 95%, Mp: 163 -164°C HPLC: 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com