Optical pH sensor of fluorescein and sodium heptanesulfonate intercalated layered double hydroxide and preparation method thereof
A technology of sodium heptanesulfonate and ph sensor, which is applied in fluorescence/phosphorescence, testing pH value, and analysis using chemical indicators, etc. It can solve the problems of small assembly thickness and poor binding force, and achieve fast and strong film formation. Effect of force, good pH response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
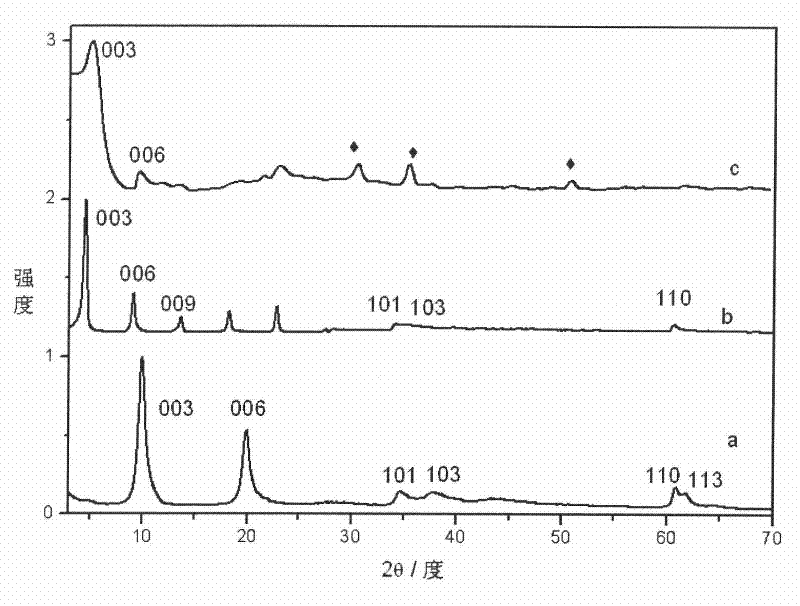
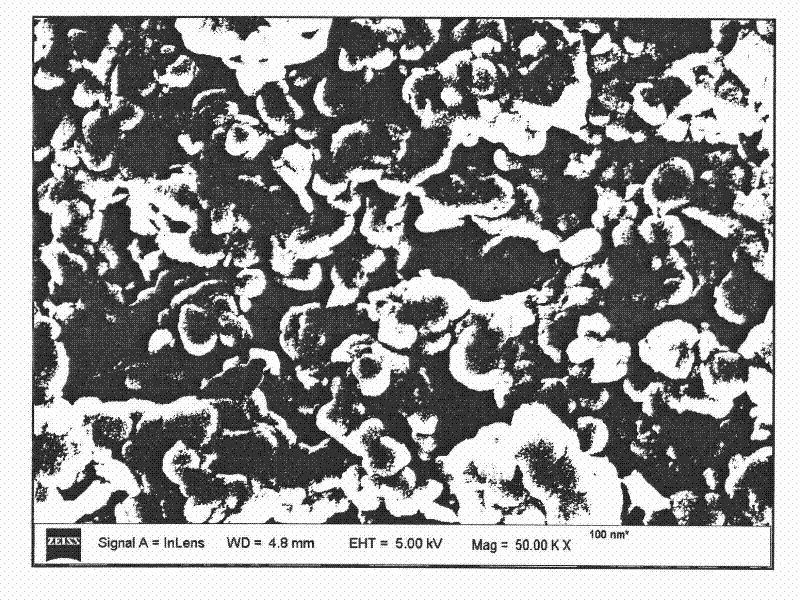
[0038] Step A: take by weighing 15.38g Mg(NO 3 ) 2 ·6H 2 O and 11.25g Al(NO 3 ) 3 9H 2 O, Mg / Al=2, dissolved in 150ml to remove CO 2 , deionized water to prepare mixed salt solution, another 45ml NH 3 ·H 2 O Adjust the above mixed solution to pH 8.5 to obtain a slurry; place the slurry in a pressure vessel and react at 140°C for 10 h to remove CO 2 , Centrifugal washing with deionized water for 4 times to obtain a slurry; use 150ml of 2g slurry to remove CO 2 , Dispersed with deionized water, prepared into a colloidal solution, sealed and stored;
[0039] Step B: Dissolve fluorescein and sodium heptanesulfonate in deCO 2 , deionized water, respectively, the concentration is 2×10 -5 mol / l and 0.01mol / l solutions;
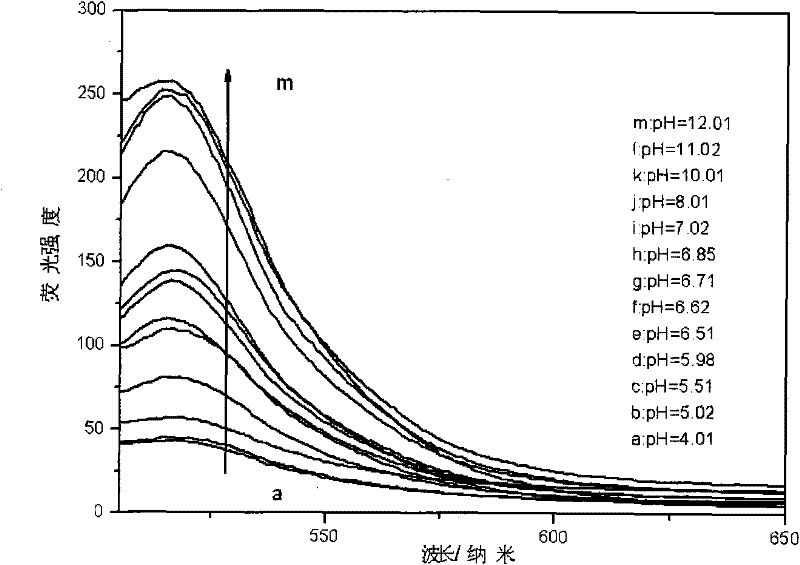
[0040] Step C: Take 15ml of the colloid solution prepared in step A, 10ml of the fluorescein solution prepared in step B, and 10ml of sodium heptanesulfonate solution in a four-necked bottle, add 150ml to remove CO 2 , deionized water; titrated with 0.2mo...
Embodiment 2
[0045] Step A: take by weighing 23.04g Mg(NO 3 ) 2 ·6H 2 O and 11.25g Al(NO 3 ) 3 9H 2 O, Mg / Al=3, dissolved in 150ml to remove CO 2 , deionized water to prepare mixed salt solution, another 45ml NH 3 ·H 2 O adjust the above mixed solution to pH 8 to obtain a slurry; place the slurry in a pressure vessel and react at 130°C for 10 h to remove CO 2 , Centrifugal washing with deionized water 3 times to obtain a slurry; use 160ml of 3g slurry to remove CO 2 , Dispersed with deionized water, prepared into a colloidal solution, sealed and stored;
[0046] Step B: CO removal with fluorescein and sodium heptanesulfonate 2 , deionized water, and were prepared with a concentration of 2×10 -5 mol / l and 0.01mol / l solutions;
[0047] Step C: Take 20ml of the colloid solution prepared in step A, 15ml of the fluorescein solution prepared in step B, and 15ml of sodium heptanesulfonate solution in a four-necked bottle, add 150ml to remove CO 2 , deionized water; titrated with 0.2mo...
Embodiment 3
[0052] Step A: take by weighing 17.64g Zn(NO 3 ) 2 ·6H 2 O and 11.25g Al(NO 3 ) 3 9H 2 O, Zn / Al=2, dissolved in 150ml to remove CO 2 , deionized water to prepare mixed salt solution, another 45ml NH 3 ·H 2O adjust the above mixed solution to pH 7 to obtain a slurry; place the slurry in a pressure vessel and react at 120°C for 10 h to remove CO 2 , Centrifugal washing with deionized water 3 times to obtain a slurry; use 160ml of 3g slurry to remove CO 2 , Dispersed with deionized water, prepared into a colloidal solution, sealed and stored;
[0053] Step B: Dissolve fluorescein and sodium heptanesulfonate in deCO 2 , deionized water, respectively, the concentration is 5×10 -5 mol / l and 0.02mol / l solutions;
[0054] Step C: Take 16ml of colloidal solution prepared in step A, 8ml of fluorescein solution prepared in step B, and 8ml of sodium heptanesulfonate solution in a four-necked bottle, add 150ml to remove CO 2 , deionized water; titrated with 0.2mol / l NaOH soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com