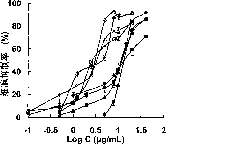
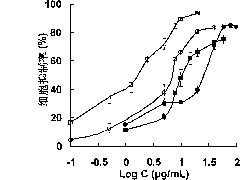
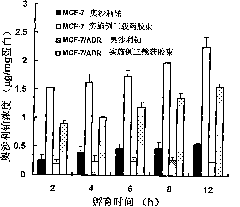
Oxaliplatin chitosan-stearic acid grafting micelle and application
A technology of oxaliplatin and stearic acid, which is applied in the field of preparation of oxaliplatin chitosan-stearic acid graft micelles, can solve the problems of not many, difficult polymer micelles, etc., and achieve the improvement of cell Toxicity, improve clinical anti-tumor efficacy, increase the effect of intracellular drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1) Preparation of low molecular weight chitosan
[0020] Get 90g of chitosan (95% degree of deacetylation) with a commercially available molecular weight of 450kDa, add it to 3000mL of 1.25% (v / v) hydrochloric acid aqueous solution, under the temperature condition of 55~60°C, after swelling for 2 hours, Add 5% cellulase (w / w), and degrade at a temperature of 55-60°C. Control the reaction temperature and time to obtain low molecular weight chitosan. The resulting degradation reaction solution was fractionated by ultrafiltration using 50Kda and 10Kda ultrafiltration membranes (Biomax-10, Millipore Co., USA), and the ultrafiltrate with a molecular weight of 10-50Kda was taken for freeze-drying. Gel permeation chromatography was used to measure chitosan molecular weight, using TSK-gel G3000SW chromatographic column, 0.1mol / L sodium acetate (pH6.0) as mobile phase, with molecular weight 0.73, 5.9, 11.8, 47.3, 212.0, 788.0Kda glucose The elution curve of the polysaccharide ...
Embodiment 2
[0029] 1) Preparation of low molecular weight chitosan
[0030] Get 90g of chitosan (95% degree of deacetylation) with a commercially available molecular weight of 450kDa, add it to 3000mL of 1.25% (v / v) hydrochloric acid aqueous solution, under the temperature condition of 55~60°C, after swelling for 2 hours, Add 5% cellulase (w / w), and degrade at a temperature of 55-60°C. Control the reaction temperature and time to obtain low molecular weight chitosan. The resulting degradation reaction solution was fractionated by ultrafiltration using 50Kda and 10Kda ultrafiltration membranes (Biomax-10, Millipore Co., USA), and the ultrafiltrate with a molecular weight of 10-50Kda was taken for freeze-drying. Gel permeation chromatography was used to measure chitosan molecular weight, using TSK-gel G3000SW chromatographic column, 0.1mol / L sodium acetate (pH6.0) as mobile phase, with molecular weight 0.73, 5.9, 11.8, 47.3, 212.0, 788.0Kda glucose The elution curve of the polysaccharide ...
Embodiment 3
[0039] 1) Preparation of low molecular weight chitosan
[0040] Get 90g of chitosan (95% degree of deacetylation) with a commercially available molecular weight of 450kDa, add it to 3000mL of 1.25% (v / v) hydrochloric acid aqueous solution, under the temperature condition of 55~60°C, after swelling for 2 hours, Add 5% cellulase (w / w), and degrade at a temperature of 55-60°C. Control the reaction temperature and time to obtain low molecular weight chitosan. The resulting degradation reaction solution was fractionated by ultrafiltration using 50Kda and 10Kda ultrafiltration membranes (Biomax-10, Millipore Co., USA), and the ultrafiltrate with a molecular weight of 10-50Kda was taken for freeze-drying. Gel permeation chromatography was used to measure chitosan molecular weight, using TSK-gel G3000SW chromatographic column, 0.1mol / L sodium acetate (pH6.0) as mobile phase, with molecular weight 0.73, 5.9, 11.8, 47.3, 212.0, 788.0Kda glucose The elution curve of the polysaccharide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com