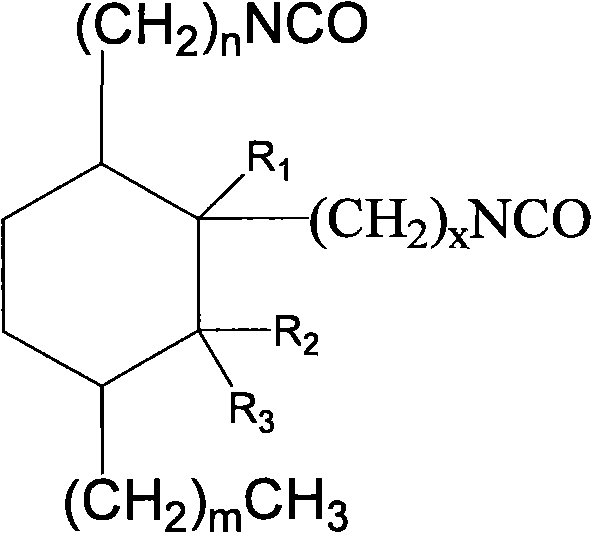
Alicyclic diisocyanate and preparation method and purposes thereof
A diisocyanate and alicyclic technology, which is applied in the field of C23-38 alicyclic diisocyanate and its preparation, can solve the problems of serious environmental pollution, high cost, difficult production of diisocyanate, etc., and has low requirements and low cost. , Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1.C 23 Cycloaliphatic diisocyanate
[0053] step 1:
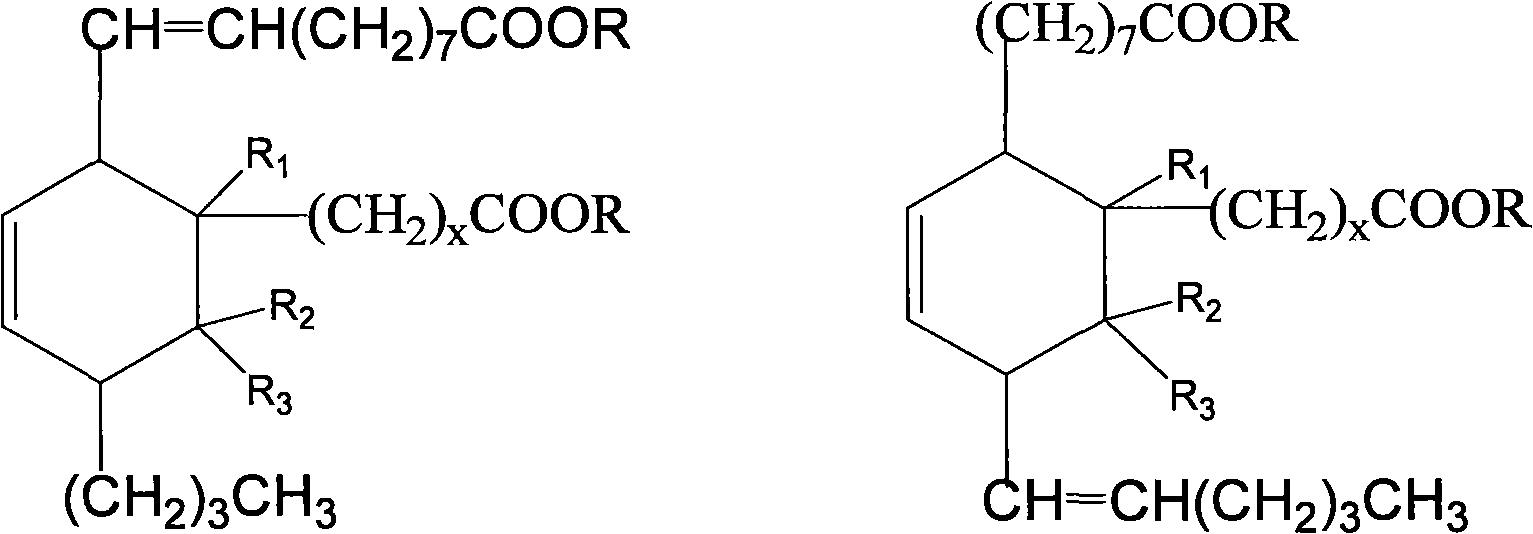
[0054] Add 500g (1.7mol) methyl oleate, 146.2g (1.7mol) methyl acrylate and 1.5g hydroquinone to a 2000mL pressure reactor, replace the air in the kettle with nitrogen, and heat to 170°C at a pressure of 0.5MPa , reacted for 3.5 hours to obtain dibasic acid methyl ester crude product, its structural formula is:
[0055]
[0056] Step 2:
[0057] Thermometer is housed in 1000mL, reflux condenser, add the dibasic acid methyl ester that step generates on 378g (1.0mol), 5g tributyl phosphate in the container of air duct and tail gas absorption device. Heat and stir to 130°C, slowly pass in dry ammonia gas, the flow rate of ammonia gas is 600-800mL / min., raise the temperature to 320°C after about 3 hours, and keep warm for 10 hours. Sampling and analysis of the acid value <1.0, that is the end of the reaction, cooling down for later use.
[0058] Step 3:
[0059] The crude product obtained from the above ...
Embodiment 2
[0073] Example 2.C 24 Cycloaliphatic diisocyanate
[0074] step 1:
[0075] Add 500g (1.7mol) methyl oleate, 170.0g (1.7mol) α-methyl methacrylate and 1.5g hydroquinone in the 2000mL pressure reactor, replace the air in the kettle with nitrogen, and the pressure is 0.4MPa, Heating to 180°C, and reacting for 3 hours to obtain the crude dibasic acid methyl ester, whose structural formula is:
[0076]
[0077] Step 2:
[0078] Thermometer is housed in 1000mL, reflux condenser, add the dibasic acid ester that 392g (1.0mol) last step generates, 5g tributyl phosphate in the container of air duct and tail gas absorption device. Heat and stir to 140°C, slowly pass in dry ammonia gas, the flow rate of ammonia gas is 600-800mL / min., heat up to 300°C after about 2 hours, and keep warm for 8 hours. Sampling and analysis of the acid value <1.0, that is the end of the reaction, cooling down for later use.
[0079] Step 3:
[0080] The crude product obtained from the above reaction ...
Embodiment 3
[0094] Example 3.C 24 Cycloaliphatic diisocyanate
[0095] step 1:
[0096] Add 500g (1.7mol) methyl oleate, 170.0g (1.7mol) β-methyl methacrylate (methyl crotonate) and 1.5g hydroquinone to a 2000mL pressure reactor, and replace the air in the kettle with nitrogen , the pressure is normal pressure, heated up to 320°C, and reacted for 10 hours to obtain the crude dibasic acid ester, whose structural formula is:
[0097]
[0098] Step 2:
[0099] In the 1000mL thermometer that is equipped with, reflux condenser, the four-necked flask of gas guide tube and tail gas absorption device, add the product that 392g (1.0mol) last step generates, 5g tributyl phosphate. Heat and stir to 100°C, slowly pass in dry ammonia gas, the flow rate of ammonia gas is 600-800mL / min., raise the temperature to 280°C after about 5 hours, and keep the reaction for 12 hours. Sampling and analysis of the acid value <1.0, that is the end of the reaction, cooling down for later use.
[0100] Step 3:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com