Process for production of phenol derivatives substituted with iodine at ortho position
A phenol derivative and ortho-position technology are applied in the production field of phenol derivatives substituted by iodine at the ortho-position, can solve the problems of high cost, complicated steps, increase the burden of purification and the like, and achieve the effect of high yield and low cost
Inactive Publication Date: 2010-08-18
MITSUBISHI GAS CHEM CO INC
View PDF3 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Therefore, only half the amount of iodine is used for 3-(3,5-diiodo-4-hydroxyphenyl)propionic acid, and a step for collecting and reusing the remaining iodine is required, thus causing these steps to become a complex and costly problem
Also increases the burden of purification due to the production of large amounts of the intermediate by-product 3-(3-iodo-4-hydroxyphenyl)propanoic acid
(4) On the other hand, there have been cases where the iodination of an aromatic compound nucleus using iodine and hydrogen peroxide has been used for the iodination of biphenyl (for example, refer to Patent Document 2), however, it has not been disclosed In the case of phenol derivatives
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
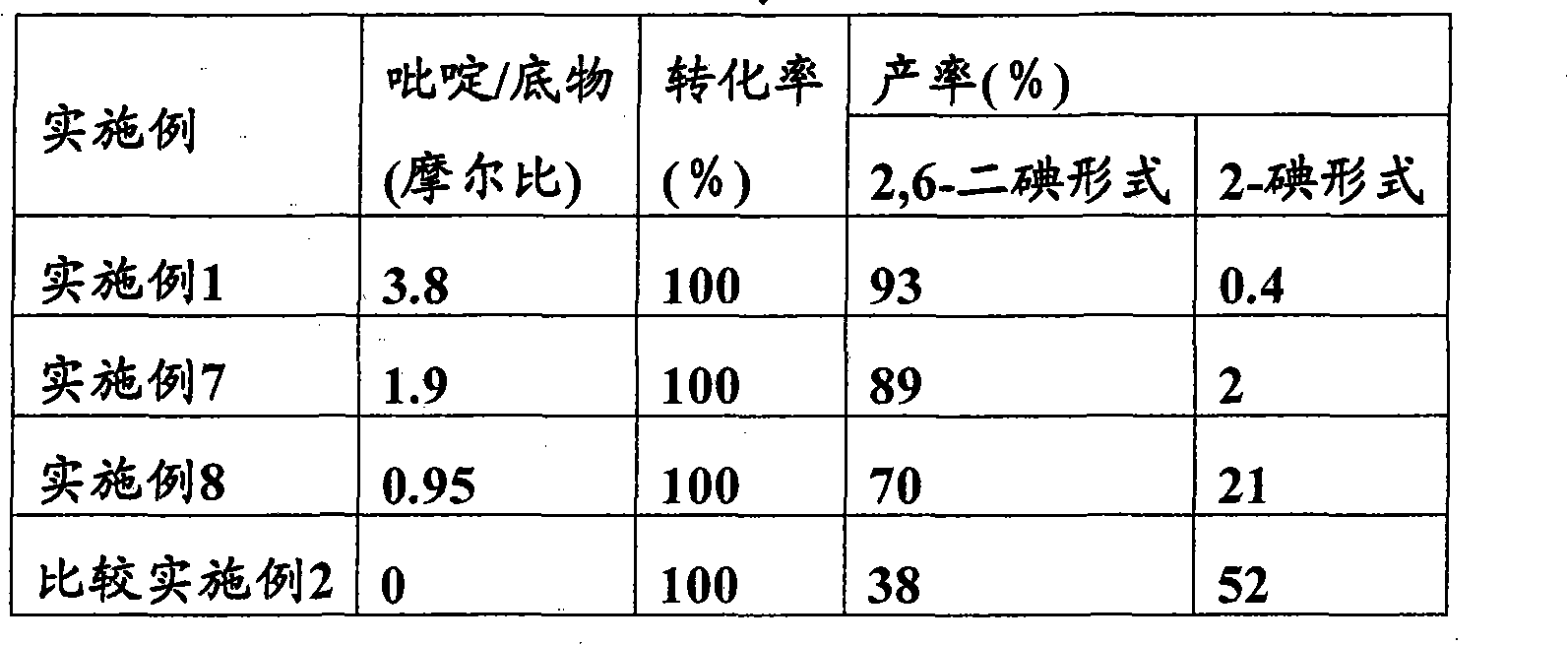
To provide a process for producing 2-iodophenol derivatives or 2,6-diiodophenol derivatives by iodinating phenol derivatives at ortho position which can dispense the recovery of iodine and makes it possible to produce the objective iodinated derivatives at low cost, in high yield, and with high quality. High-quality 2-iodophenol derivatives or 2,6-diiodophenol derivatives can be obtained in high yield by reacting a phenol derivative with molecular iodine in the presence of a pyridine and either hydrogen peroxide or iodic acid as oxidizing agent through efficient iodination with a nearly theoretical amount of iodine based on the starting phenol derivative.
Description
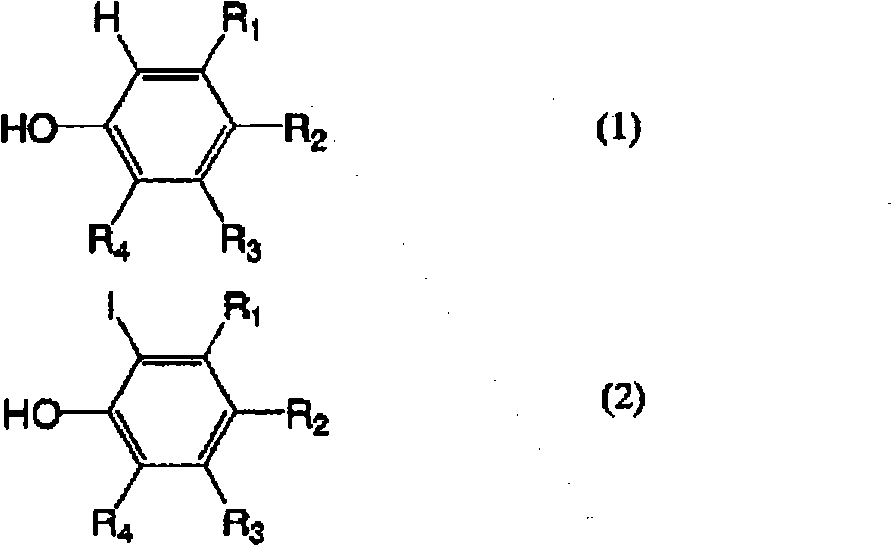
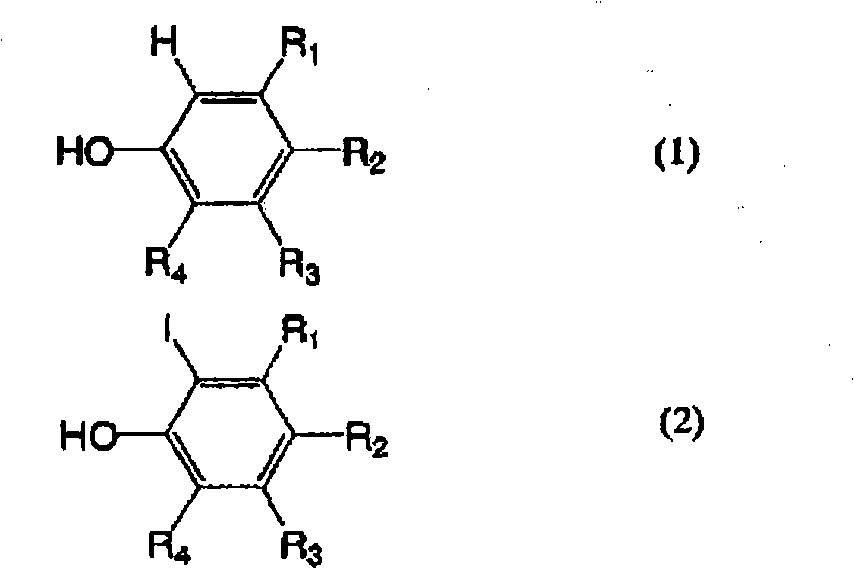
technical field The present invention relates to a method for selectively producing 2-iodophenol derivatives or 2,6-diiodophenol derivatives by iodination of phenol derivatives whose one or two substituents at the ortho position are hydrogen. 2-iodophenol derivatives or 2,6-diiodophenol derivatives are compounds useful as raw materials in medicine or agricultural chemistry. For example, examples of 2-iodophenol derivatives include 2-iodo-3-hydroxy-4-methoxybenzonitrile, which is 4-amino-6,7-dimethoxy-2 An important intermediate raw material of -(5-methylsulfonylamino-1,2,3,4-tetrahydroisoquinolin-2-yl)-5-(2-pyridyl)quinazoline. Examples of 2,6-diiodophenol derivatives include 3-(3,5-diiodo-4-hydroxyphenyl)propanoic acid, which is a 3,5- An important intermediate raw material of diiodothyronine. Background technique Generally, (1) a method of reacting an iodine or potassium triiodide aqueous solution with an alkaline aqueous solution is generally known as a method for iodi...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C45/63C07C47/575C07C51/363C07C59/56C07C59/64C07C65/03C07C65/21C07C253/30C07C255/54C07B61/00
CPCC07C59/56C07C253/30C07C51/42C07C45/63C07C51/363C07C47/575C07C65/03C07C65/21C07C255/54
Inventor 田中一夫
Owner MITSUBISHI GAS CHEM CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com