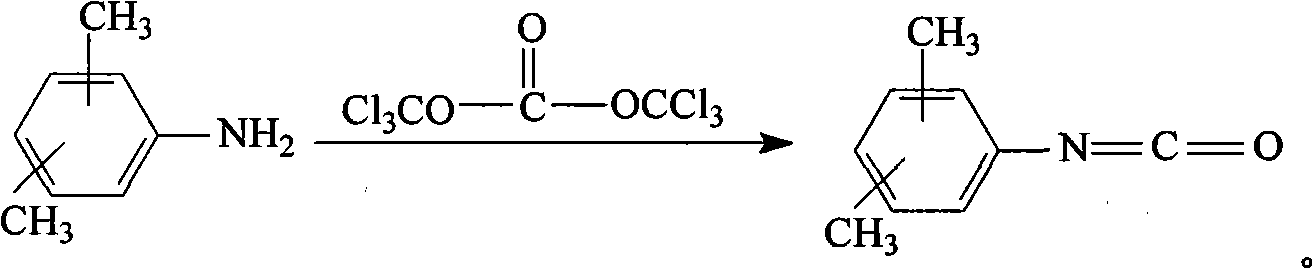
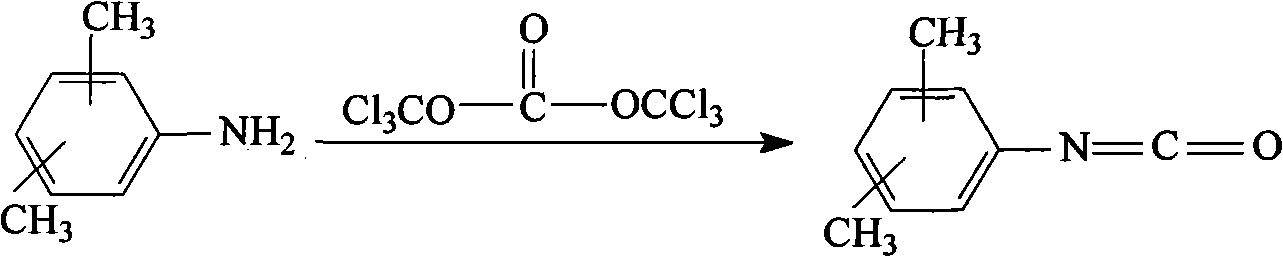Method for preparing dimethylphenyl isocyanate
A technology of dimethylphenylisocyanate and phenylisocyanate, which is applied in the field of preparation of dimethylphenylisocyanate, can solve problems such as no substantial progress, achieve low production costs, improve the operating environment, and avoid separation operations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Weigh 27.9g of 3,4-dimethylaniline and 29.7g of solid phosgene and dissolve them in 100mL solvent 1,2-dichloroethane respectively, then control the temperature at 0-5°C, and slowly dissolve the dimethylaniline solution under stirring Add it dropwise to the solid phosgene solution, after the addition is complete, heat up to 75-80°C for reflux reaction for 5 hours. After the reaction, the solvent was removed, and the substrate was distilled under reduced pressure to obtain the product 3,4-dimethylphenylisocyanate with a yield of 81.97% and a product content of 96.91% (HPLC method).
Embodiment 2
[0017] Weigh 26.7g of 3,5-dimethylaniline and 29.7g of solid phosgene and dissolve them in 100mL of solvent 1,2-dichloroethane respectively, then control the temperature at 0-5°C, slowly dissolve the dimethylaniline solution under stirring Add it dropwise to the solid phosgene solution, after the addition is complete, heat up to 75-80°C and reflux for 4 hours. After the reaction, the solvent was removed, and the substrate was distilled under reduced pressure to obtain the product 3,5-dimethylphenylisocyanate with a yield of 91.04% and a product content of 98.56% (HPLC method).
Embodiment 3
[0019] Weigh 24.2g of 2,6-dimethylaniline and 29.7g of solid phosgene and dissolve them in 100mL of solvent 1,2-dichloroethane respectively, then control the temperature at 0-5°C, slowly dissolve the dimethylaniline solution under stirring Add it dropwise to the solid phosgene solution, after the addition is complete, heat up to 75-80°C for reflux reaction for 3 hours. After the reaction, the solvent was removed, and the substrate was distilled under reduced pressure to obtain the product 2,6-dimethylphenylisocyanate with a yield of 90.52% and a product content of 90.91% (HPLC method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com