Supported gold catalyst and preparation method thereof
A gold catalyst, supported technology, applied in the field of supported gold catalyst with low load and high activity and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
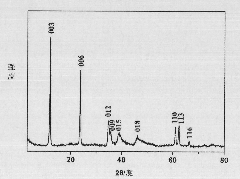
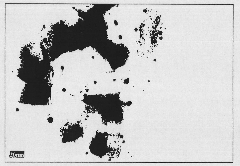
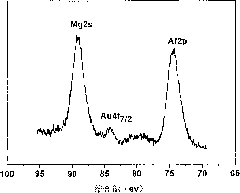
Image
Examples
Embodiment 1
[0023] A: Weigh 61.54g Mg(NO 3 ) 2 ·6H 2 O and 45.02g Al(NO 3 ) 3 9H 2 O was dissolved in deionized water to make 300mL mixed salt solution; then weighed 23.04g NaOH and 25.44g NaOH 2 CO 3 Dissolve in deionized water to make 300mL mixed alkali solution; add the above two mixed solutions into the total back-mixed liquid membrane reactor at the same time, adjust the slit width between the reactor rotor and stator to 0.02mm, the working voltage to 100V, and the rotor speed 4000rpm, add the obtained mixed slurry into the crystallization kettle and stir, keep the temperature of the mixed slurry in the kettle at 95-105°C for reflux crystallization for 6 hours, and obtain the magnesium-aluminum hydrotalcite precursor slurry; the magnesium-aluminum hydrotalcite precursor The slurry was centrifuged and washed to a pH of 7.5, and dried at 90°C for 24 hours to obtain magnesium-aluminum hydrotalcite;
[0024] B: Measure 1.75ml of chloroauric acid solution with a concentration of 10...
Embodiment 2
[0028] A: with embodiment 1;
[0029] B: Measure 3.525ml of chloroauric acid solution with a concentration of 10g / L, 20ml of deionized water, and 0.756g of urea, and add the above substances into a 50ml single-necked flask respectively; after standing for three minutes to dissolve, weigh the magnesium-aluminum hydrotalcite Add 1.0 g to the above single-necked flask; place the single-necked flask in a heat-collecting constant-temperature heating magnetic stirrer, adjust the temperature of the heat-collecting constant-temperature heating magnetic stirrer to 80°C, and keep the constant temperature for 5 hours, then turn off the heat-collecting constant temperature heating magnetic force Stirrer, aging for 2 hours; centrifuge and wash the aged solution until there is no chloride ion in the solution, take out the sample, put it in a 90°C electric constant temperature blast drying oven for drying, and take it out after 12 hours;
[0030] C: with embodiment 1;
[0031] D: Carry out ...
Embodiment 3
[0033] A: with embodiment 1;
[0034] B: Measure 17.5ml of chloroauric acid solution with a concentration of 10g / L, 100ml of deionized water, and 2.523g of urea, respectively add the above substances into a 250ml single-necked flask, let it stand for three minutes to dissolve, and then weigh the magnesium-aluminum hydrotalcite Add 1.0g into a single-necked flask; place the single-necked flask in a collector-type constant-temperature heating magnetic stirrer, adjust the temperature of the collector-type constant-temperature heating magnetic stirrer to 80°C, and keep the constant temperature for 5 hours, then turn off the collector-type constant-temperature heating magnetic stirrer Centrifuge and wash the aged solution until there is no chloride ion in the solution, take out the sample, put it in a 90°C electric constant temperature blast drying oven for drying, and take it out after 12 hours;
[0035] C: with embodiment 1;
[0036] D: Carry out application performance test to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com