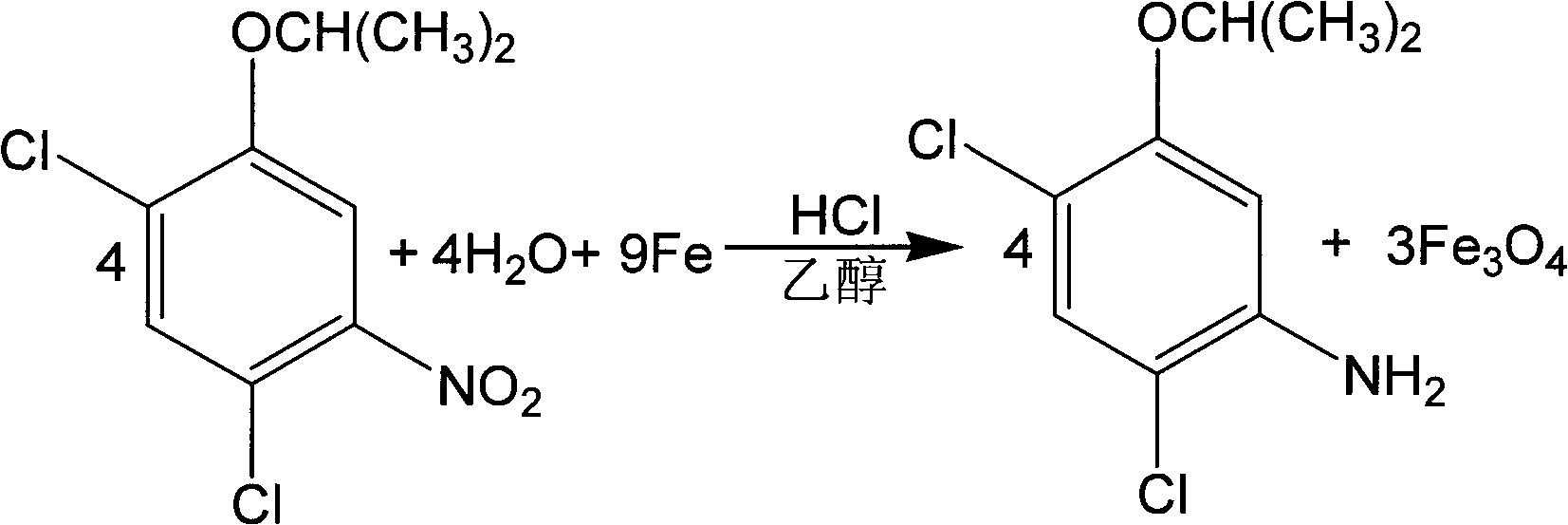
Synthesis method of 2,4-dichloro-5-isopropoxy aniline
A technology of propoxyaniline and a synthesis method, applied in 2 fields, can solve the problems of cumbersome process operation, large amount of three wastes, poor product quality, etc., and achieves the effects of reducing production cost, improving yield, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
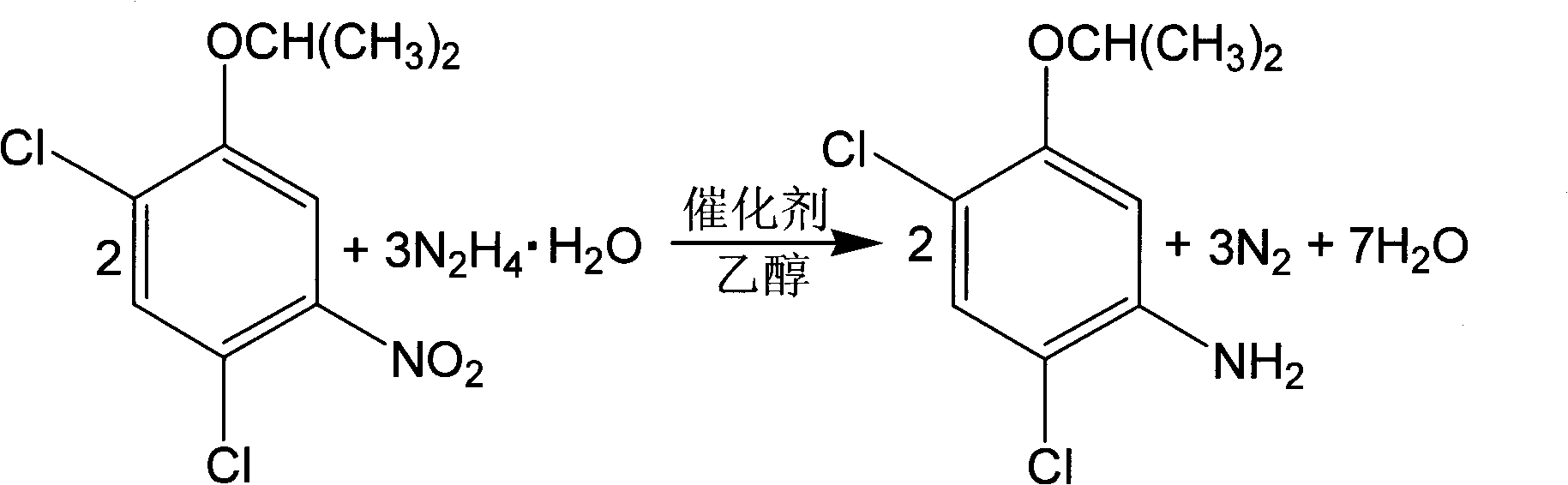
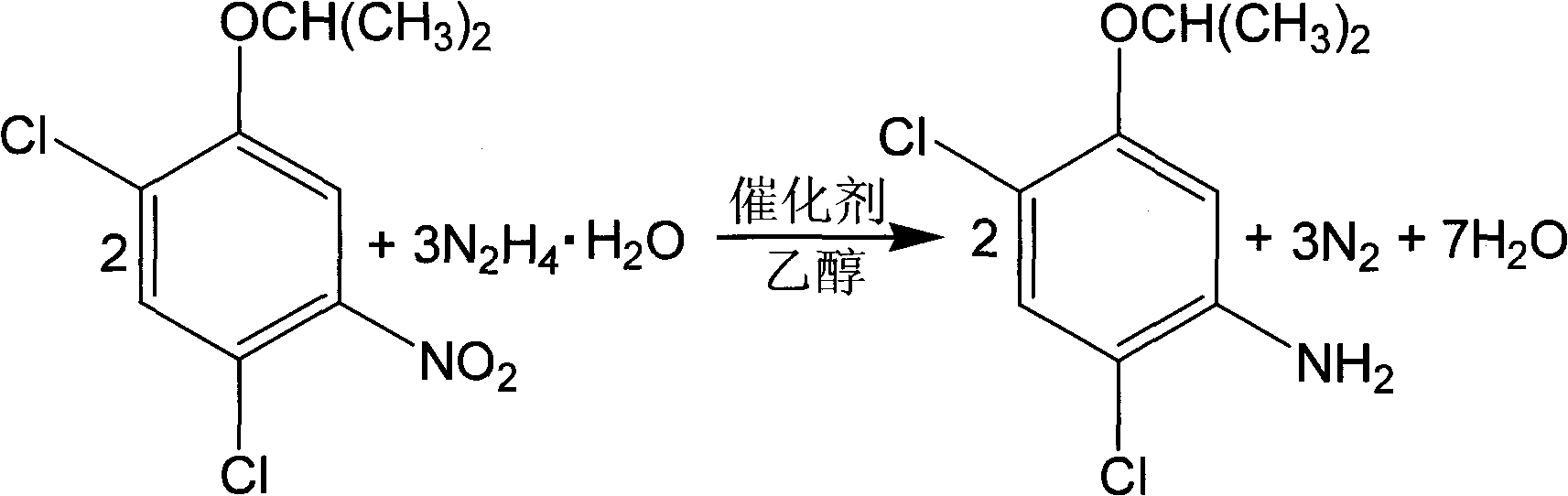
[0025] With 2,4-dichlorophenol as the starting material, 100g of 2,4-dichloro-5-isopropoxynitrobenzene (content 91.6% ) into the four-necked bottle, which is respectively equipped with a thermometer, an electric stirring device, a condenser and a constant pressure funnel, and the 150mL specification is 95% (V / V) ethanol and 20g composite catalyst {10g gac, 5g Fe(OH) 3 , 5g Al(OH) 3} into the four-necked flask, stirred for 0.5 hours, and began to heat. When the temperature rose to 65°C, 45.8g of hydrazine hydrate (80%) was slowly dripped into the four-necked flask from the constant pressure funnel, and the dropping time was 1 ~3 hours, continue to react at 60~80°C for 2~6 hours after dropping, stop the reaction, cool down to 30°C, and obtain the filtrate by suction filtration, which is 2,4-dichloro-5-isopropoxyaniline solution . The weight of the filtrate was 254.5 g, the content of 2,4-dichloro-5-isopropoxyaniline in the filtrate was 30.5%, and the reaction yield was 96.3%....
Embodiment 2
[0027] With 2,4-dichlorophenol as the starting material, 100g of 2,4-dichloro-5-isopropoxynitrobenzene (content 91.6% ) into the four-necked bottle, which is respectively equipped with a thermometer, an electric stirring device, a condenser and a constant pressure funnel, and the 150mL specification is 95% (V / V) ethanol and 4g composite catalyst {2g gac, 1g Fe(OH) 3 , 1g Al(OH) 3} into the four-necked flask, stirred for 0.5 hours, and began to heat. When the temperature rose to 65°C, 45.8g of hydrazine hydrate (80%) was slowly dripped into the four-necked flask from the constant pressure funnel, and the dropping time was 1 ~3 hours, continue to react at 60~80°C for 2~6 hours after dropping, stop the reaction, cool down to 30°C, and obtain the filtrate by suction filtration, which is 2,4-dichloro-5-isopropoxyaniline solution . The weight of the filtrate was 259.8 g, the content of 2,4-dichloro-5-isopropoxyaniline in the filtrate was 28.3%, and the reaction yield was 91.2%. ...
Embodiment 3
[0029] With 2,4-dichlorophenol as the starting material, 100g of 2,4-dichloro-5-isopropoxynitrobenzene (content 91.6% ) into the four-necked bottle, which is respectively equipped with a thermometer, an electric stirring device, a condenser and a constant pressure funnel, and the 150mL specification is 95% (V / V) ethanol and 60g composite catalyst {30g gac, 15g Fe(OH) 3 , 15g Al(OH) 3} into the four-necked flask, stirred for 0.5 hours, and began to heat. When the temperature rose to 65°C, 45.8g of hydrazine hydrate (80%) was slowly dripped into the four-necked flask from the constant pressure funnel, and the dropping time was 1 ~3 hours, continue to react at 60~80°C for 2~6 hours after dropping, stop the reaction, cool down to 30°C, and obtain the filtrate by suction filtration, which is 2,4-dichloro-5-isopropoxyaniline solution . The weight of the filtrate was 249.8 g, the content of 2,4-dichloro-5-isopropoxyaniline in the filtrate was 30.8%, and the reaction yield was 95.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com