Synthesis method of 1-methyl-3-trifluoromethyl pyrazol
A technology of trifluoromethylpyrazole and a synthetic method, applied in directions such as organic chemistry, can solve the problems of high cost, inability to carry out at room temperature, large proportion of isomers, etc., to achieve improved yield and avoid excessive waste. The effect of liquid generation and reduction of volatilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
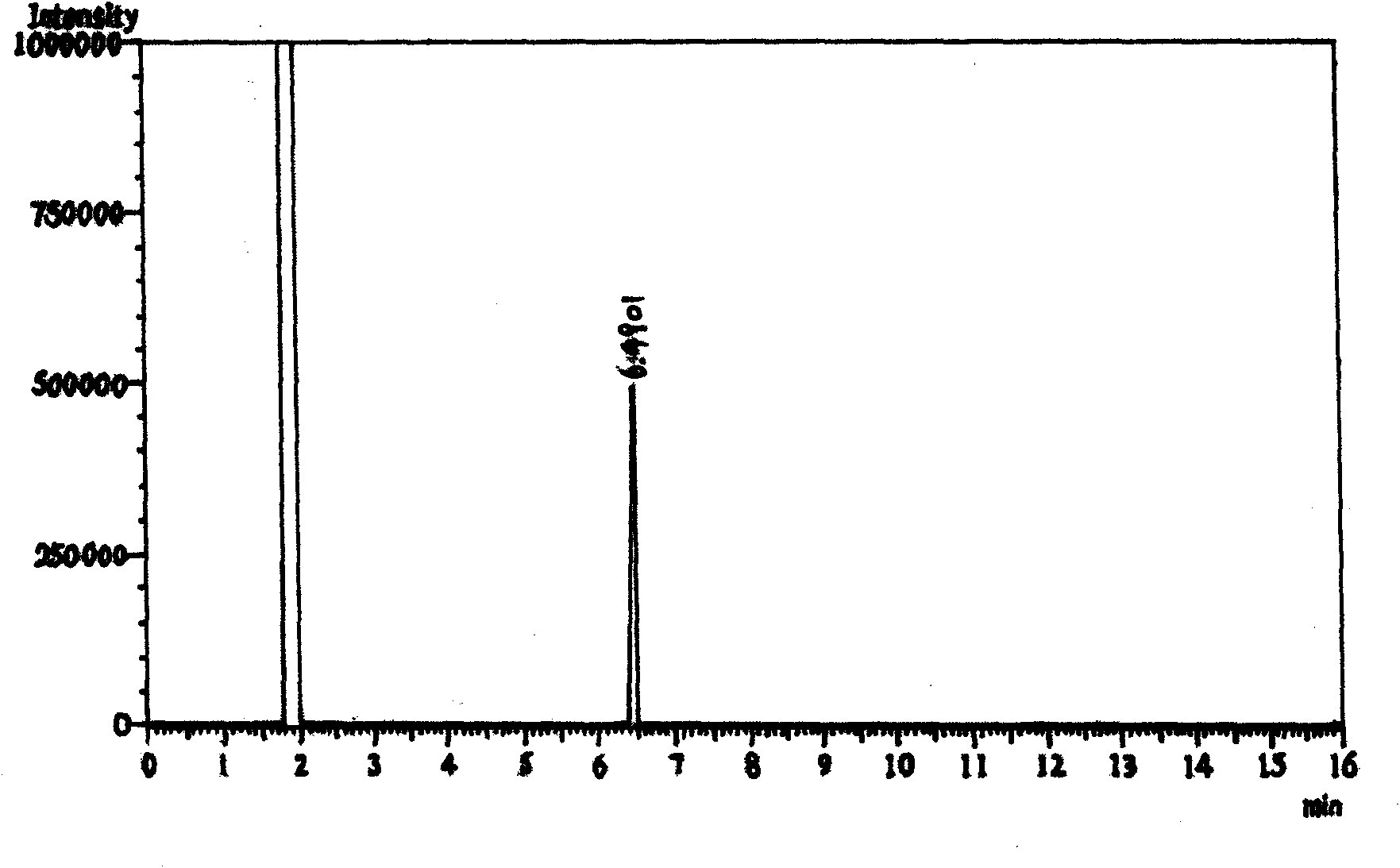
[0049] Add 7.1g of sodium hydroxide and 140mL of water into a 250mL four-neck flask, stir slowly until the solid dissolves, then add 9.9g of methylhydrazine into the flask, cool down to 0°C with an ice bath, and slowly add 30g of Tris Fluoroacetylvinyl ethyl ether, after the dropwise addition, slowly return to room temperature and react for 0.5 hours. During monitoring, use 1N HCl to adjust to PH=4~5, extract with dichloromethane, monitor by GC, ratio of 1-methyl-3-trifluoromethylpyrazole to 1-methyl-5-trifluoromethylpyrazole 13:87 (see attached image 3 ). Post-treatment, the reaction solution was extracted three times with 80 mL of dichloromethane, washed three times with 50 mL of saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent and the ethanol generated to obtain 20.85 g of 1-methyl-3-trifluoro Methylpyrazole, colorless liquid. Yield: 77.8%, Purity: 99.3% (GC).
Embodiment 2
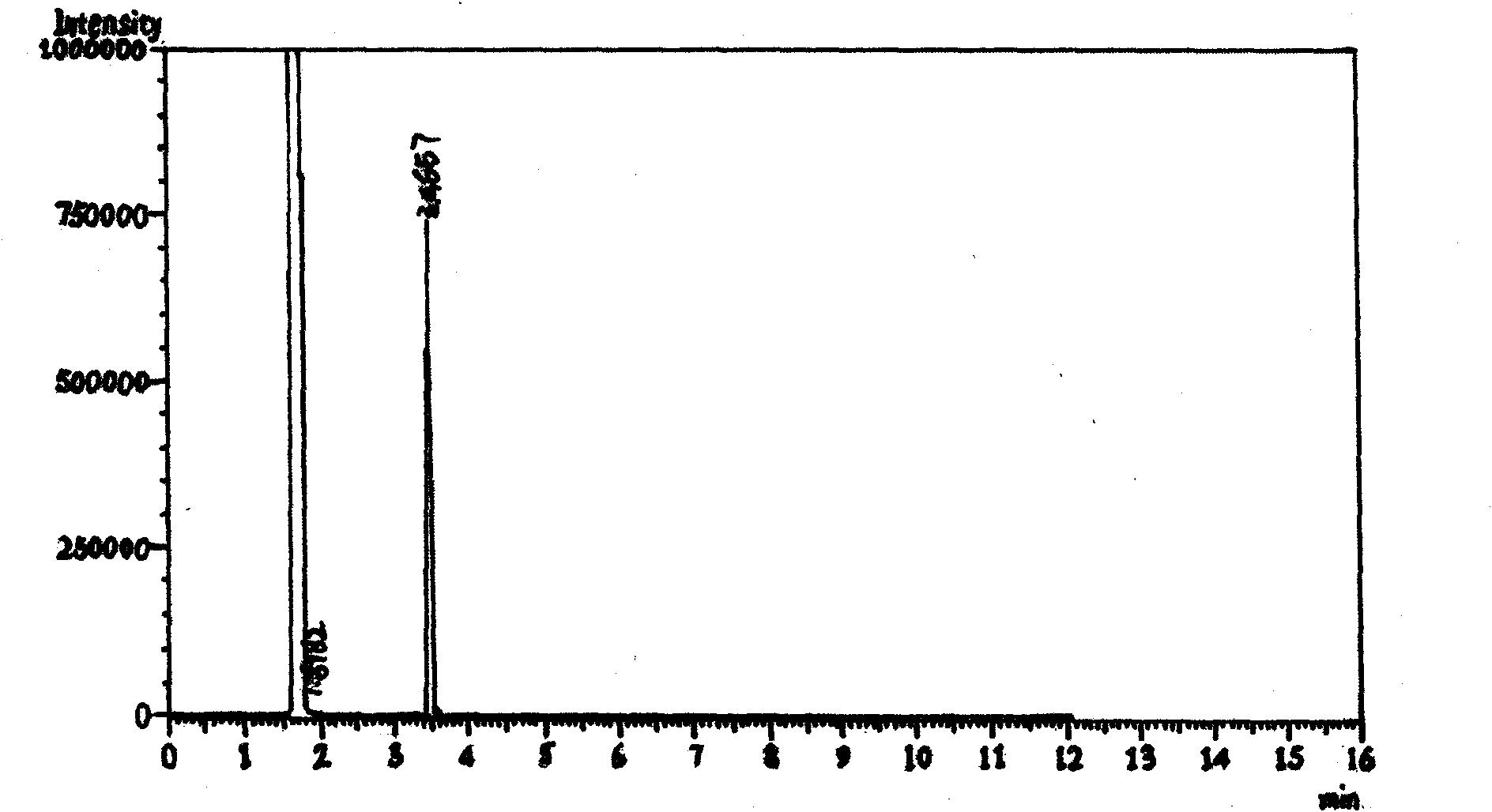
[0051] Add 3.55g of sodium hydroxide and 140mL of water into a 250mL four-neck flask, stir slowly until the solid dissolves, then add 9.9g of methylhydrazine into the flask, cool down to 0°C with an ice bath, and slowly add 30g of trihydrazine dropwise at 0°C Fluoroacetylvinyl ethyl ether, after the dropwise addition, slowly return to room temperature and react for 0.5 hours. During monitoring, use 1N HCl to adjust to PH=4~5, extract with dichloromethane, monitor by GC, ratio of 1-methyl-3-trifluoromethylpyrazole to 1-methyl-5-trifluoromethylpyrazole It is 12:88. After treatment, the reaction solution was extracted three times with 80 mL of dichloromethane, washed three times with 50 mL of saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent and the ethanol generated to obtain 20.45 g of 1-methyl-3-trifluoro Methylpyrazole, colorless liquid. Yield: 76.4%, Purity: 99.1% (GC).
Embodiment 3
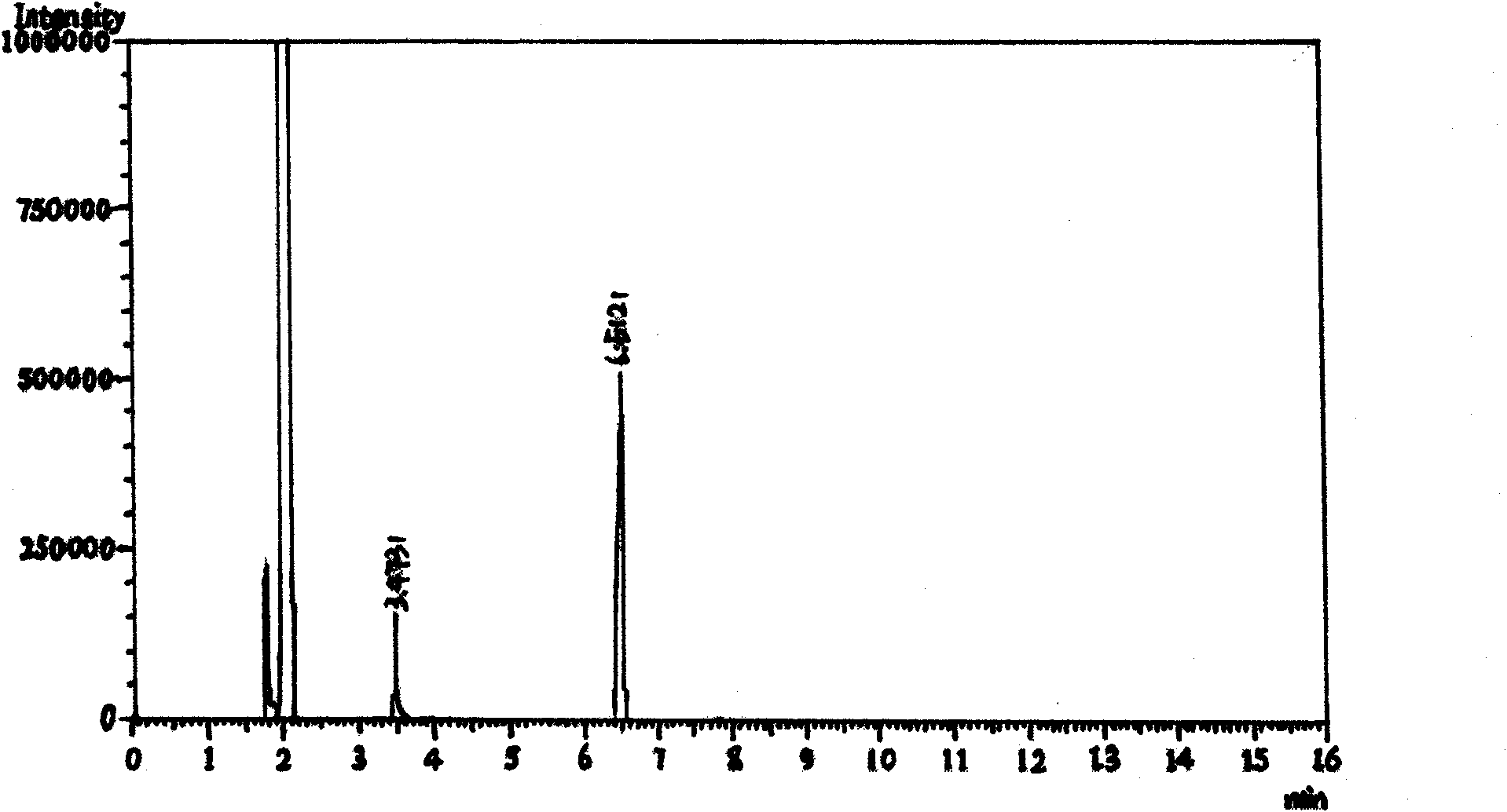
[0053]Add 10.0g of potassium hydroxide and 140mL of water into a 250mL four-neck flask, stir slowly until the solid dissolves, then add 9.9g of methylhydrazine into the flask, cool down to 0°C with an ice bath, and slowly add 30g of Fluoroacetylvinyl ethyl ether, after the dropwise addition, slowly return to room temperature and react for 0.5 hours. During monitoring, use 1N HCl to adjust to PH=4~5, extract with dichloromethane, monitor by GC, ratio of 1-methyl-3-trifluoromethylpyrazole to 1-methyl-5-trifluoromethylpyrazole It is 12:88. Post-treatment, the reaction solution was extracted three times with 80 mL of dichloromethane, washed three times with 50 mL of saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent and the ethanol generated to obtain 21.05 g of 1-methyl-3-trifluoro Methylpyrazole, colorless liquid. Yield: 78.6%, Purity: 98.9% (GC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com