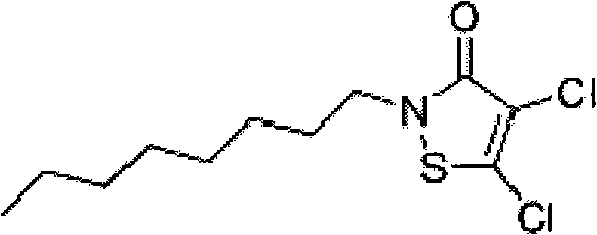Preparation method of isothiazolinone biocide mildewcide
The technology of isothiazolinone, bactericidal and antifungal agent is applied in the field of preparation of isothiazolinone type bactericidal antifungal agent, and can solve the problems of high volatility, high equipment corrosion, and high safety hazard.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
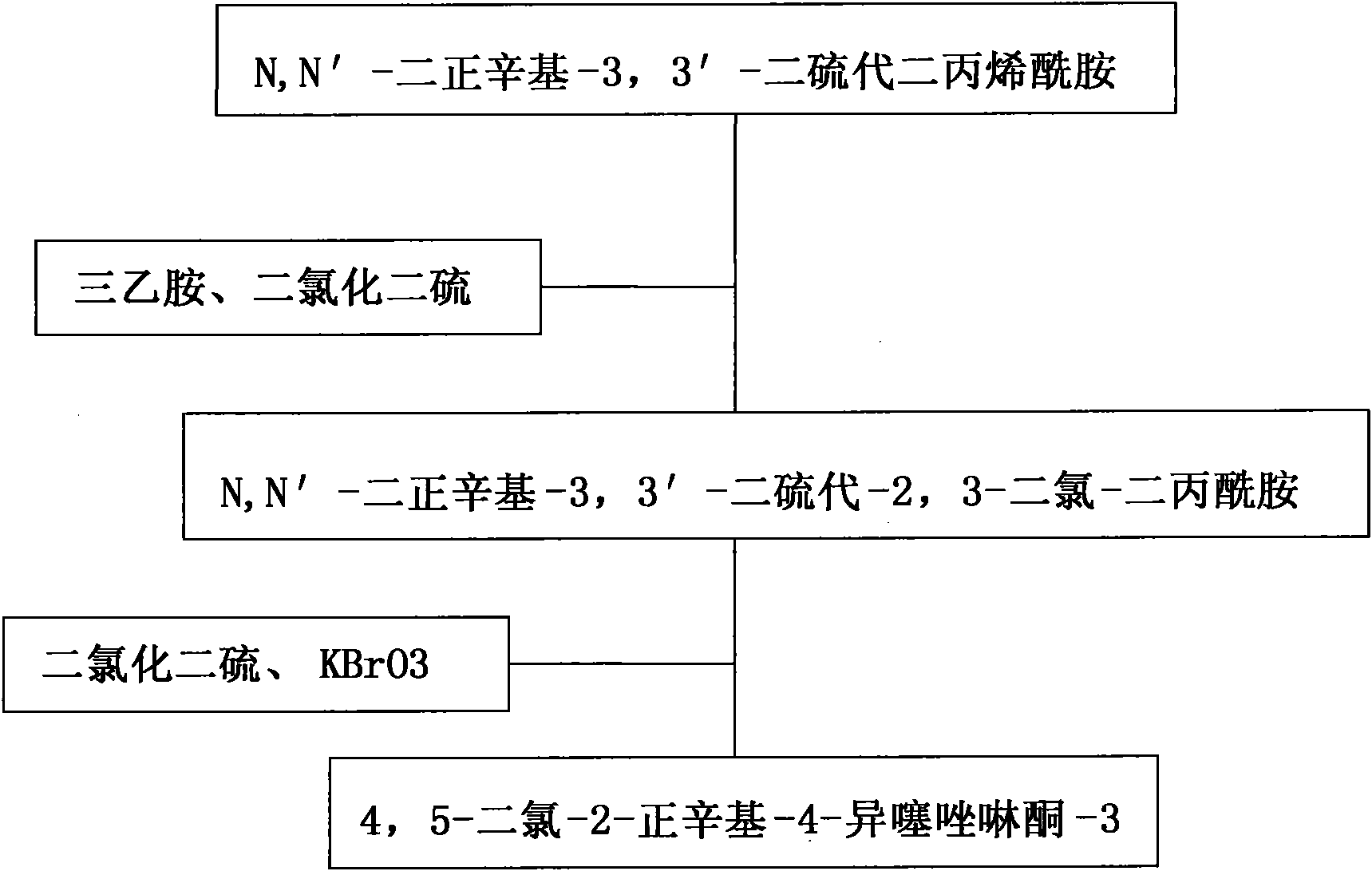
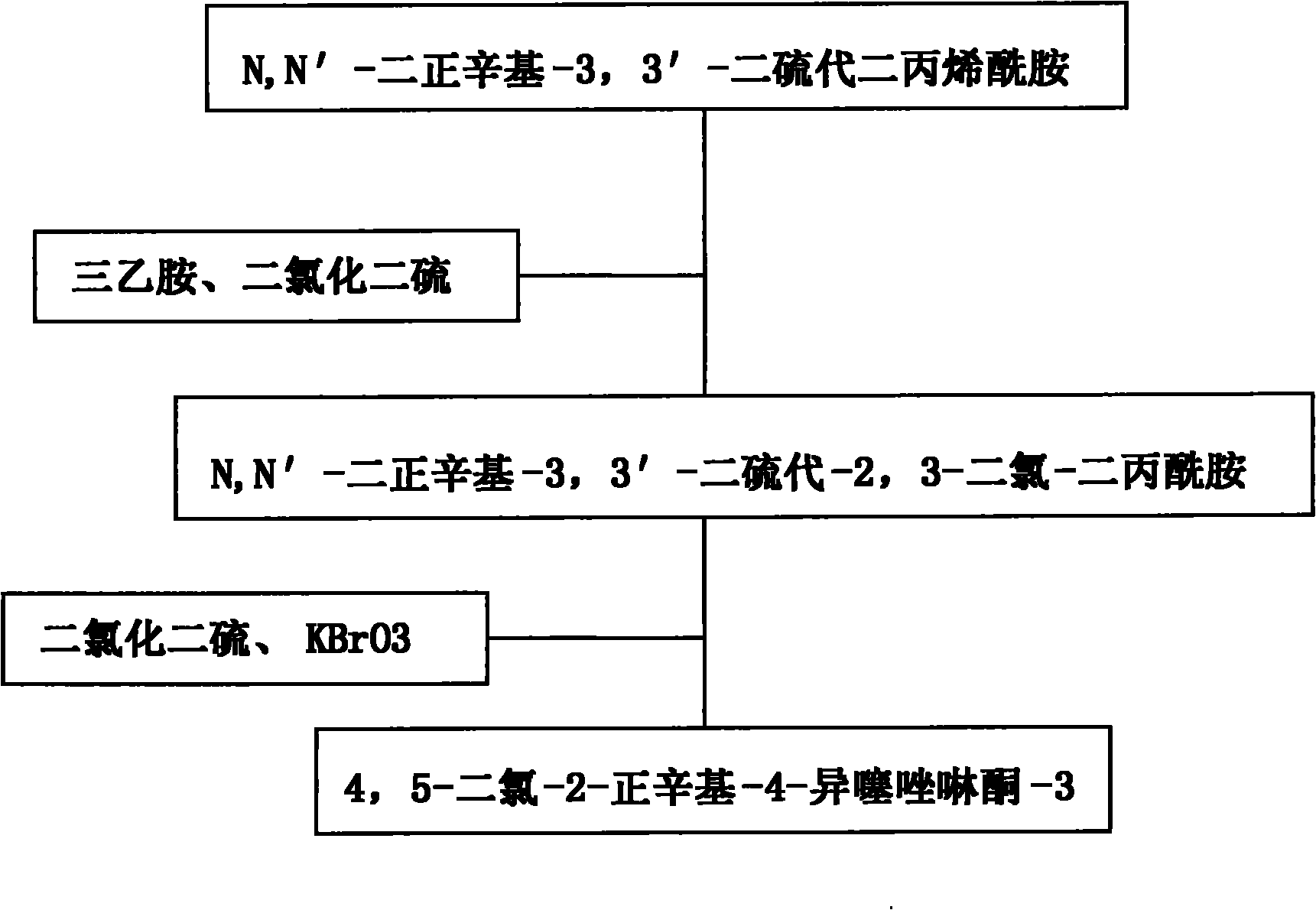
[0017] a. N, N'-di-n-octyl-3,3'-dithiobisacrylamide: disulfide dichloride: potassium bromate: chlorobenzene: triethylamine in a molar ratio of 1:2:0.1:2 : 1.5 mixed and put into an enamel kettle with a reflux condenser, heat preservation reaction at 100°C for 13 hours, wherein N,N'-di-n-octyl-3,3'-dithiobisacrylamide is based on chlorobenzene Solvent, under the condition that triethylamine exists, react with disulfur dichloride to generate N, N'-di-n-octyl-3,3'-dithio-2,3-dichloro-dipropionamide, and then The N,N'-di-n-octyl-3,3'-dithio-2,3-dichloro-dipropionamide is subjected to a ring-closing reaction with disulfide dichloride in the presence of potassium bromate;
[0018] b. Put the above reaction product into a stainless steel kettle, purify with ethanol at a temperature of 34°C, and recrystallize in an ice-salt water bath at -12°C to obtain 4,5-dichloro-2-n-octyl Base-4-isothiazolinone-3.
Embodiment 2
[0020] a. N, N'-di-n-octyl-3,3'-dithiobisacrylamide: disulfide dichloride: potassium bromate: chlorobenzene: triethylamine in a molar ratio of 1:3:0.3:2.5 : 2.5 mixed and put into an enamel kettle with a reflux condenser, heat preservation reaction at 125°C for 15 hours, wherein N,N'-di-n-octyl-3,3'-dithiobisacrylamide is based on chlorobenzene Solvent, under the condition that triethylamine exists, react with disulfur dichloride to generate N, N'-di-n-octyl-3,3'-dithio-2,3-dichloro-dipropionamide, and then The N,N'-di-n-octyl-3,3'-dithio-2,3-dichloro-dipropionamide is subjected to a ring-closing reaction with disulfide dichloride in the presence of potassium bromate;
[0021] b. Put the above reaction product into a stainless steel kettle, purify with ethanol at a temperature of 40°C, and recrystallize in an ice-salt bath at -10°C to obtain 4,5-dichloro-2-n-octyl Base-4-isothiazolinone-3.
Embodiment 3
[0023] a. N, N'-di-n-octyl-3,3'-dithiobisacrylamide: disulfide dichloride: potassium bromate: chlorobenzene: triethylamine in a molar ratio of 1: 2.5: 0.2: 2.2 : 2 mixed and put into an enamel kettle with a reflux condenser, heat preservation reaction for 18.5 hours at 120°C, wherein N, N'-di-n-octyl-3,3'-dithiobisacrylamide is based on chlorobenzene Solvent, under the condition that triethylamine exists, react with disulfur dichloride to generate N, N'-di-n-octyl-3,3'-dithio-2,3-dichloro-dipropionamide, and then The N,N'-di-n-octyl-3,3'-dithio-2,3-dichloro-dipropionamide is subjected to a ring-closing reaction with disulfide dichloride in the presence of potassium bromate;
[0024] b. Put the above reaction product into a stainless steel kettle, purify it with ethyl acetate at a temperature of 45°C, and recrystallize it in an ice-salt water bath at -15°C to obtain 4,5-dichloro-2- n-Octyl-4-isothiazolinone-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com