Preparation method of photoresponse hydrogel containing azo monomers
A light-responsive, hydrogel technology, applied in the field of photochemical synthesis, can solve the problems of cumbersome photo-responsive hydrogel synthesis methods, and achieve the effects of mild reaction conditions, mild reaction conditions and simple steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Preparation of 4-sulfonic acid-4'acryloyloxyazobenzene hydrogel at a temperature of -40°C and an azo monomer
[0018] Combine HEA (1.5g), PEGDA-600 (0.15g), N,N methylene bisacrylamide (0.15g), camphor kun (CQ) and ethyl p-N,N-dimethylaminobenzoate (EDMAB) ) Each (0.075g) mixed solution was sonicated for 10 minutes, and 0.015g of 4-sulfonic acid-4'acryloyloxyazobenzene was added respectively. Adjust the temperature to -40°C in a low-temperature polymerization box, and irradiate it with a visible light (50mw) for 5 minutes. The prepared colloid was rinsed with water 5 times and placed in a vacuum oven at 40°C for 24 hours.
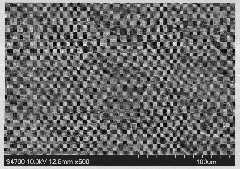
[0019] The prepared microstructure difference is as figure 1 Shown. The structural formula of the azo monomer is shown in (I).
[0020]
Embodiment 2
[0021] Example 2: Preparation of 2-chloro-4-sulfonic acid-4'acryloyloxyazobenzene hydrogel at a temperature of 0°C and an azo monomer
[0022] (1) Sonicate the mixed solution of HEA (1.5g), PEGDA-400 (0.075g), N, N methylene bisacrylamide (0.075g), and TPO (0.015g) for 10 minutes, and add 2-chloro- 0.03 g of 4-sulfonic acid-4'acryloyloxy azobenzene and water (4.5 g). The temperature was adjusted to 0°C in a low-temperature polymerization box, and a visible lamp (100mw) was used to irradiate each for 10 minutes. The prepared colloid was rinsed with water 5 times and placed in a vacuum oven at 40°C for 24 hours.
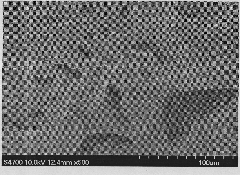
[0023] The prepared microstructure difference is as figure 2 As shown, the structural formula of the azo monomer is as shown in (II).
[0024]
Embodiment 3
[0025] Example 3: Preparation of 2-cyano-4-sulfonic acid group-2'-carboxy-4'-acryloyloxy azobenzene hydrogel at a temperature of 20°C and the azo monomer
[0026] (1) Sonicate the mixed solution of HEA (1.5g), PEGDA-200 (0.225g), N,N methylenebisacrylamide (0.225g), and TPO (0.03g) for 10 minutes, and add 2-cyano- 0.045 g of 4-sulfonic acid-2'-carboxy-4'-acryloyloxyazobenzene and water (6g). Adjust the temperature in the heating jacket to 20°C, and irradiate it with a visible light (120mw) for 20 minutes. The prepared colloid was rinsed with water 5 times and placed in a vacuum oven at 40°C for 24 hours.
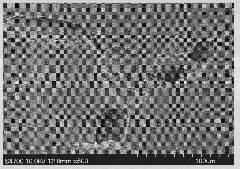
[0027] The prepared microstructure difference is as image 3 As shown, the structural formula of the azo monomer is as shown in (III).
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com