Compositions and methods for protein display on the surface of bacteria and vesicles derived therefrom and uses thereof
A protein, cell surface technology, applied in biochemical equipment and methods, chemical instruments and methods, immunoglobulins, etc., can solve the problem of not carrying signal peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
[0093] The following examples illustrate various methods of the compositions in the methods of treatment of the invention. The examples are intended to illustrate, but in no way limit the scope of the invention. EXAMPLES Example 1 - Bacterial Strains, Plasmids and Growth Conditions
[0094]The bacterial strains and plasmids used in these examples are described in Table 1. Table 1. Bacterial Strains and Plasmids
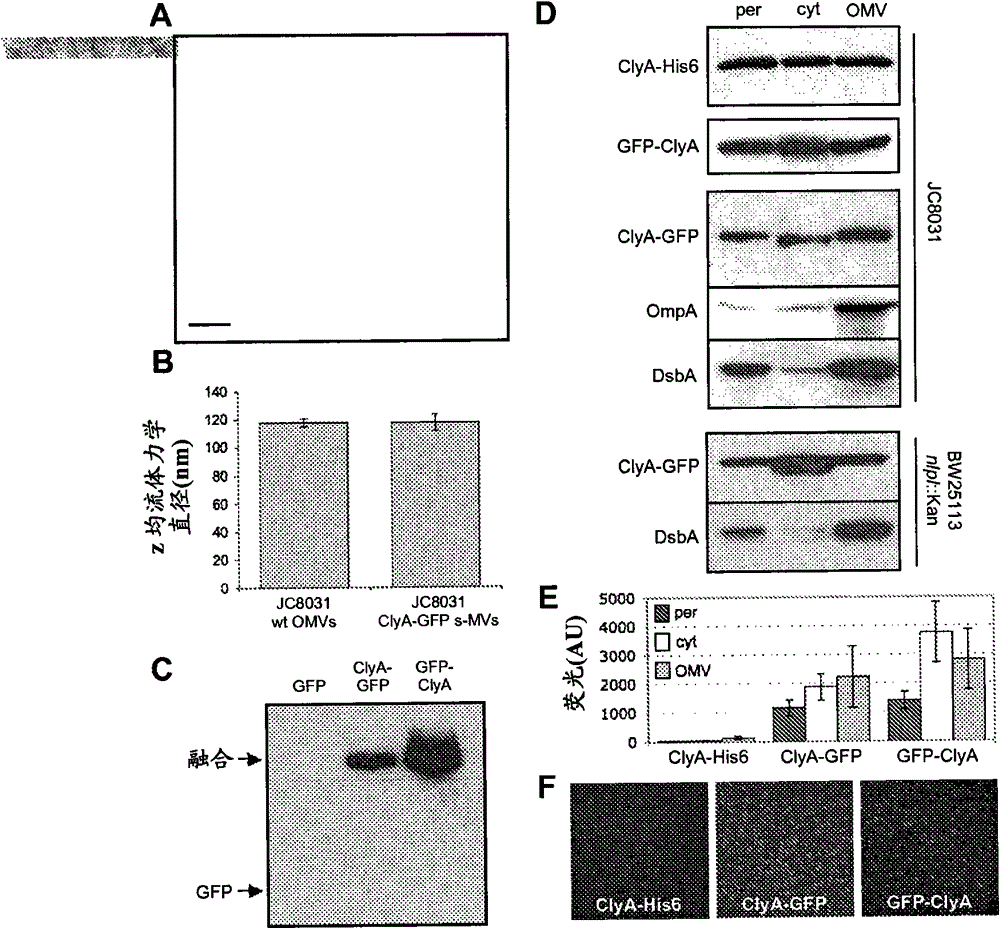
[0095] Using DHA as a donor, the dsbA::Kan allele was introduced into JC8031 via P1vir transduction to prepare cell strain JCA. The PCR-amplified clyA gene was ligated with pBAD18-Cm between SacI and XbaI sites to construct plasmid pClyA. The gene encoding gfpmut2 was inserted between the XbaI and HindIII sites (Crameri et al., "Improved Green Fluorescent Protein by Molecular Evolution Using DNA Shuffling (by using DNA shuffling molecular evolution to improve green fluorescent protein)", NatBiotechnol14: 315-9 (1996) and DeLisa et al., "GeneticAnalysisoftheTw...
Embodiment 2
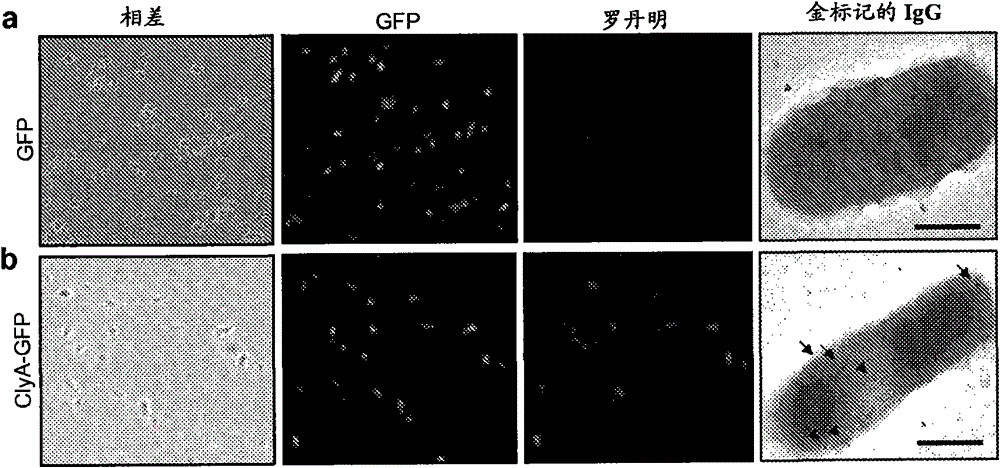
[0096] Human epithelial cervical carcinoma (HeLa) cells were obtained from the American Type Culture Collection (ATCC#CCL-2) and cultured in Dulbecco's modified Eagles minimal medium supplemented with 10% NuSerum and 1% penicillin / streptomycin (DMEM). Maintain cells at 37°C, 95% air, 5% CO 2 in a humidified atmosphere. For fluorescence microscopy experiments, cells were grown on 12-mm round glass coverslips for 2 days prior to the experiment. Example 3 - Subcellular Fractionation
Embodiment 3
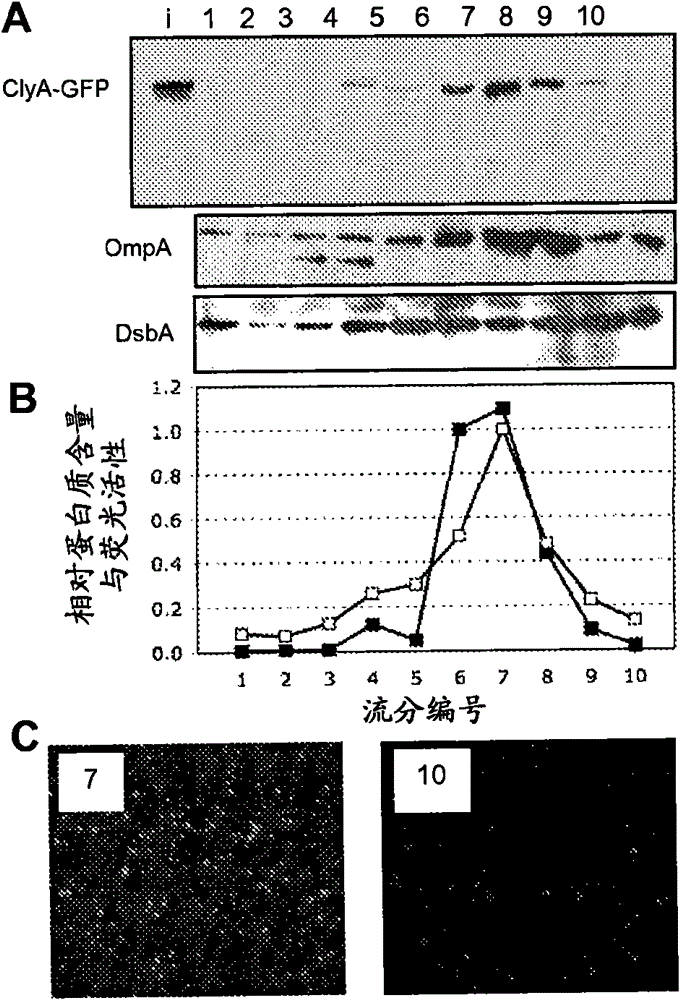
[0097] By cold osmotic shock procedure (coldosmotic shock procedure) (Kim et al., "Twin-Arginine Translocation of Active Human Tissue Plasminogen Activator in Escherichiacoli (Twin-Arginine Translocation of Active Human Tissue Plasminogen Activator in Escherichia coli)", Applied and Environmental Microbiology 71: 8451-8459 (2005 ), which is hereby incorporated by reference in its entirety), cytoplasmic and periplasmic fractions were prepared from cells expressing the fusion protein, and the residual pellet was collected as the insoluble fraction after removal of the soluble fraction. Example 4 - Isolation of Bacterial Vesicles
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com