Oxygen adsorbent, preparation method and application thereof
An oxygen adsorbent and adsorbent technology, applied in the field of preparation and oxygen adsorbent, can solve the problems of high temperature sealing, simultaneous realization, and difficulty in mechanical stability, and achieve high oxygen selectivity, fast oxygen adsorption rate, and high oxygen adsorption capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
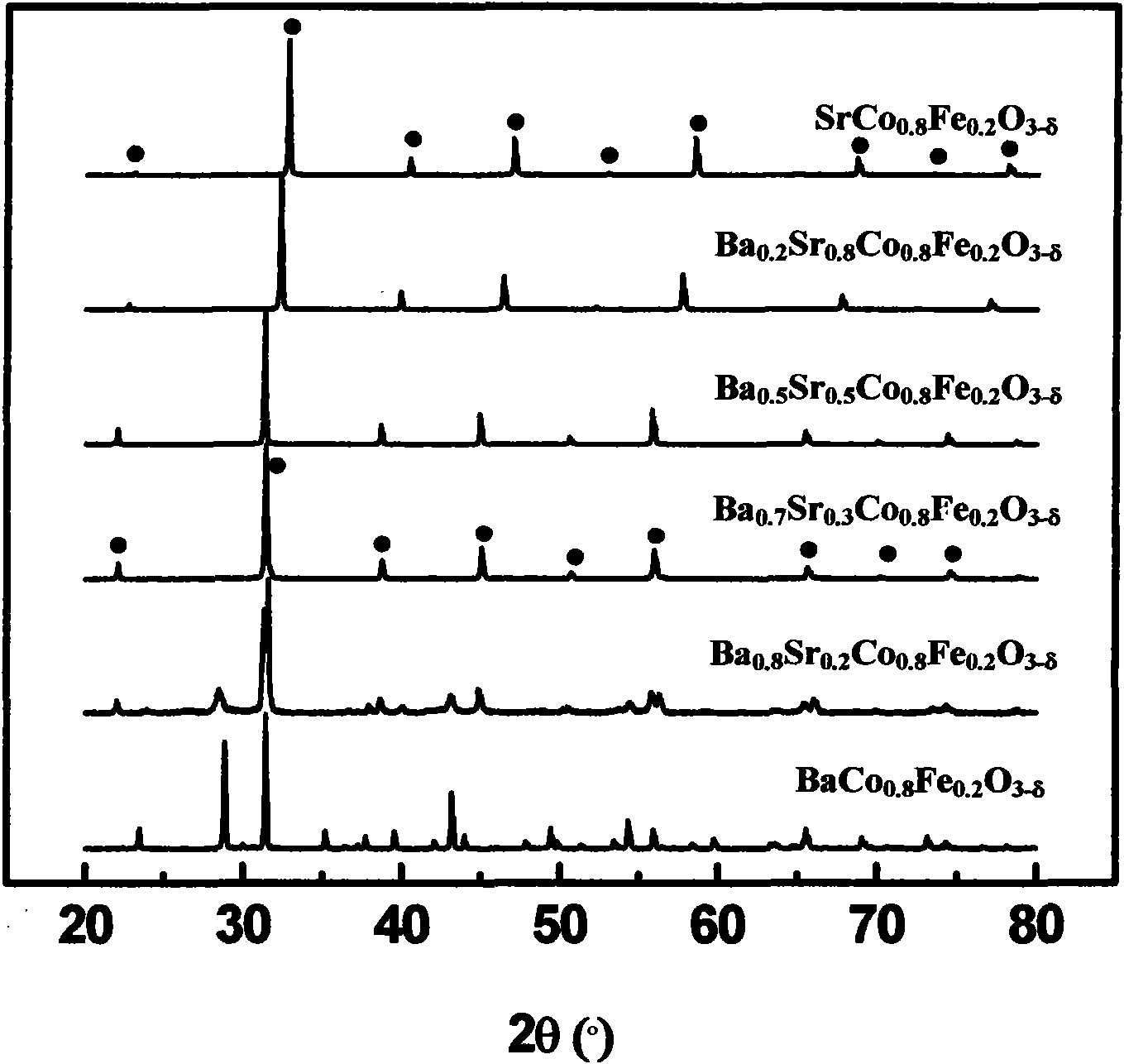
[0030] Direct mixing of nitrate solutions of all metal ions to synthesize Ba 1-z Sr z co 0.8 Fe 0.2 o 3-δ A series of composite oxides, where 0≤z≤1. The stoichiometric metal ion (Ba 2+ 、Sr 2+ 、Co 2+ , Fe 3+ ) nitrate solution in a beaker, to which EDTA and citric acid were added, and the ratio of EDTA and citric acid to the amount of total metal ions was 1:1. Then use NH 3 ·H 2 O to adjust the pH of the solution to 6.0, heat and stir the solution at a constant temperature of 80°C, and finally obtain a brown colloid with the continuous evaporation of water, decompose the colloid at 400°C to obtain the primary powder, and then make the primary powder The body was calcined at 950°C for 5 hours to obtain the composite oxide powder prepared from the mixed solution. Its XRD diagram and SEM diagram are as follows figure 1 and figure 2 . The prepared various powders were tableted and granulated under 75MPa respectively.
Embodiment 2
[0032] Synthesis of SrCo by directly mixing nitrate solutions of all metal ions 1-w Fe w o 3-δ A series of composite oxides, where 0≤w≤1. According to the stoichiometric ratio of various metal ions required for the synthesis of each oxide, the stoichiometric metal ions (Sr 2+ 、Co 2+ , Fe 3+ ) nitrate solution in a beaker, to which EDTA and citric acid were added, and the ratio of EDTA and citric acid to the amount of total metal ions was 1:1. Then use NH 3 ·H 2 O to adjust the pH of the solution to 6.0, heat and stir the solution at a constant temperature of 80°C, and finally obtain a brown colloid with the continuous evaporation of water, decompose the colloid at 400°C to obtain the primary powder, and then make the primary powder The body was calcined at 950°C for 5 hours to obtain the composite oxide powder prepared from the mixed solution. The prepared various powders were tableted and granulated under 75MPa. Example 3
Embodiment 3
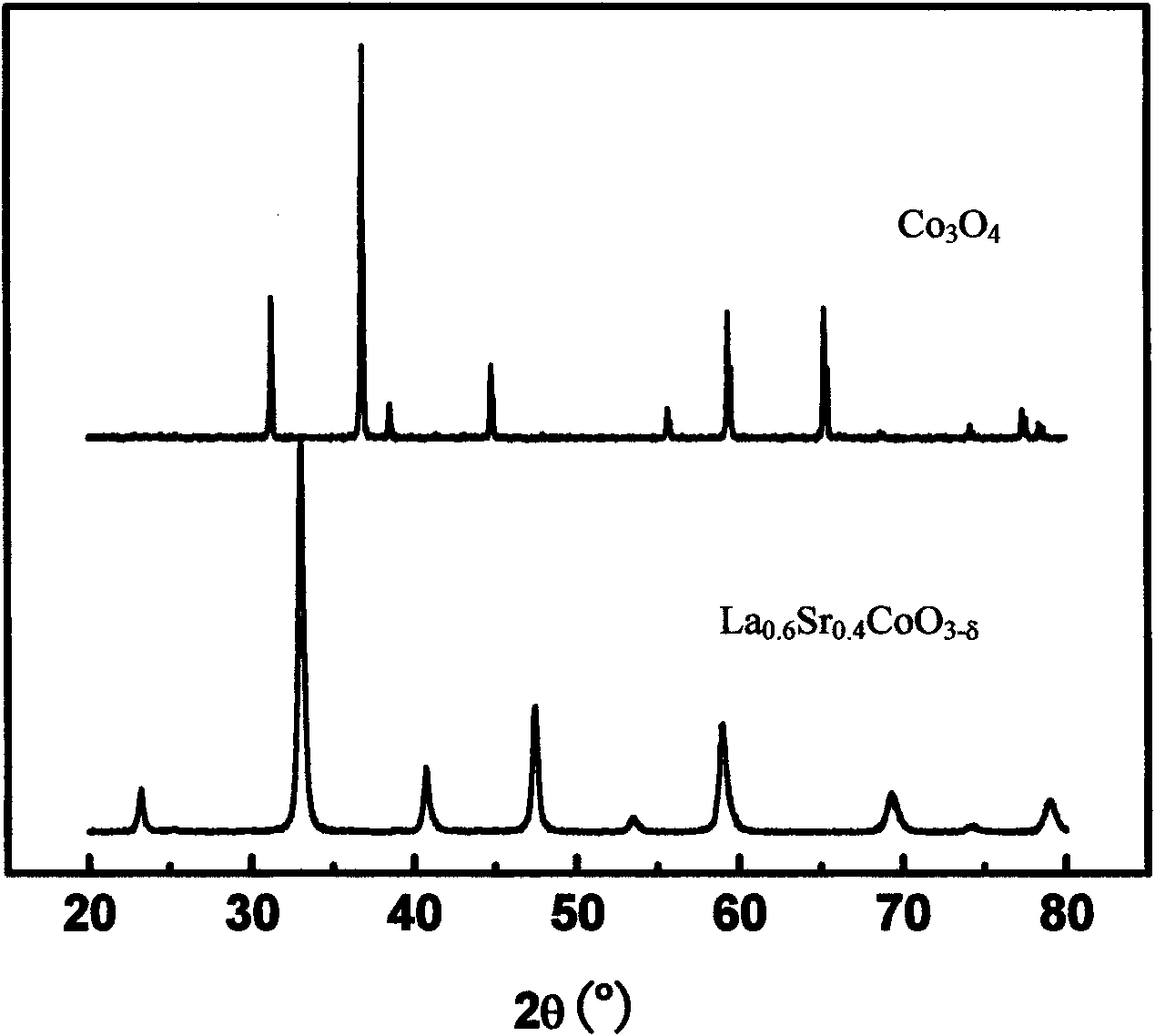
[0033] Synthesis of La by direct mixing of nitrate solutions of all metal ions 1-z Sr z CoO 3-δ A series of composite oxides, where 0≤z≤1. According to the stoichiometric ratio of the various metal ions required for the synthesis of each oxide, the stoichiometric metal ions (La 3+ 、Sr 2+ 、Co 2+ ) nitrate solution in a beaker, to which EDTA and citric acid were added, and the ratio of EDTA and citric acid to the amount of total metal ions was 1:1. Then use NH 3 ·H 2 O to adjust the pH of the solution to 6.0, heat and stir the solution at a constant temperature of 80°C, and finally obtain a brown colloid with the continuous evaporation of water, decompose the colloid at 400°C to obtain the primary powder, and then make the primary powder Calcined at 900°C for 5 hours to obtain various composite oxide powders prepared from the mixed solution, the crystal structure of which is the same as that of La 0.6 Sr 0.4 CoO 3-δ The perovskite structure, such as image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com