Method for preparing nano barium sulfate and co-producing sodium chloride
A nano-barium sulfate and sodium chloride technology, applied in the direction of alkali metal chloride, calcium/strontium/barium sulfate, etc., can solve the problems of high cost, complicated production process and equipment, and achieve low cost, high economic value, The effect of simple process and equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
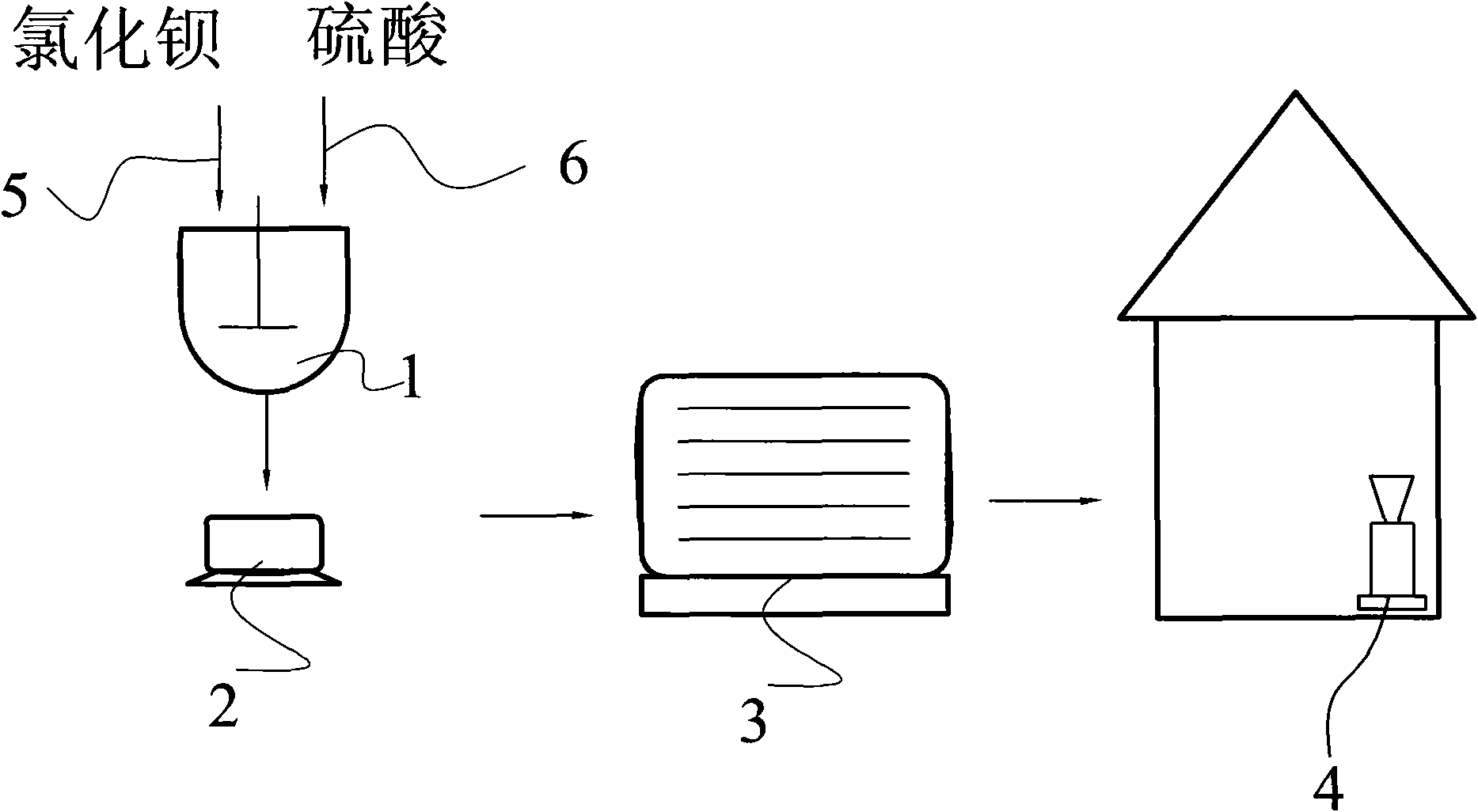
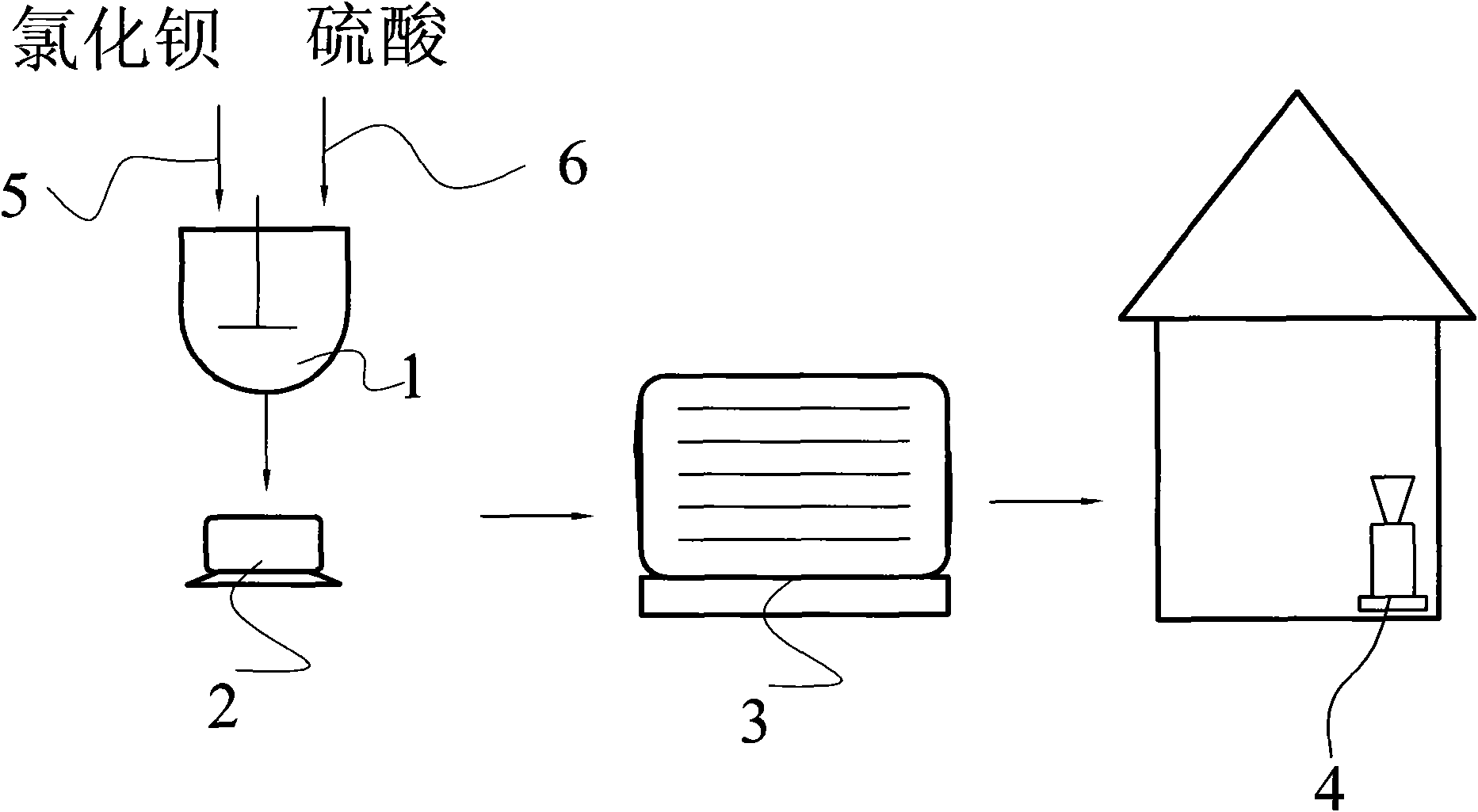
[0019] A method for preparing nano barium sulfate to co-produce sodium chloride, the preparation process is:
[0020] Dissolve 500 kilograms of barium chloride in water, inject it into the reactor through the barium chloride feeding line 5 after being treated with 4% phthalate coupling agent; then pass the dissolved sodium sulfate through the sodium sulfate feeding line 6 Inject into reactor 1, wherein the mass ratio of barium chloride and sodium sulfate in the reactor is 1:0.38 according to the mass ratio of pure substance; then stir evenly with the rotating speed of 300 rev / min, chemical reaction obtains the mixed liquid of barium sulfate and sodium chloride Mixed liquid gets filter cake and filtrate through centrifuge 2 filtration, and filter cake is dried and pulverized by drier 3, pulverizer 4 drying successively after washing, and packing obtains 312.2 kilograms of nanometer barium sulfate products; Described filtrate is through decompression distillation Get Sodium Chlo...
Embodiment 2
[0022] A method for preparing nano barium sulfate to co-produce sodium chloride, the preparation process is:
[0023] Dissolve 500 kg of barium chloride in water, treat it with 5% lignin and inject it into the reactor through the barium chloride feeding line 5; then inject the dissolved sodium sulfate into the reactor 1 through the sodium sulfate feeding line 6 , wherein barium chloride in the reactor and sodium sulfate are 1: 0.68 by the mass ratio of pure substance; Then with the rotating speed of 400 revolutions / minutes uniform stirring, chemical reaction obtains the mixed liquid of barium sulfate and sodium chloride; Mixed liquid passes through Centrifuge 2 is filtered to obtain filter cake and filtrate, and filter cake is dried and pulverized by drier 3, pulverizer 4 successively after washing, and packaging obtains 558.7 kilograms of nanometer barium sulfate products; Described filtrate obtains sodium chloride through vacuum distillation .
Embodiment 3
[0025] A method for preparing nano barium sulfate to co-produce sodium chloride, the preparation process is:
[0026] First 500 kilograms of barium chloride are dissolved in water, and after being treated with 8% surfactant (wherein stearic acid 1%, lignin 7%), inject in the reactor by barium chloride feeding line 5; Sodium sulfate is injected into reactor 1 through sodium sulfate feeding line 6, wherein barium chloride in the reactor is 1: 0.98 by the mass ratio of sodium sulfate by pure substance; Obtain the mixed liquid of barium sulfate and sodium chloride; The mixed liquid is filtered through centrifuge 2 to obtain filter cake and filtrate, and the filter cake is dried and pulverized by drying machine 3 and pulverizer 4 successively after washing, and packaging obtains 560.1 kilograms of nanometer sulfuric acid Barium product; the filtrate was distilled under reduced pressure to obtain sodium chloride.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap