Method for synthesizing stilbene compound by utilizing Kornblum oxidation reaction
A stilbene compound and oxidation reaction technology, applied in the field of synthesizing stilbene compounds by Kornblum oxidation reaction, can solve the problems of low yield, high toxicity, environmental damage and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
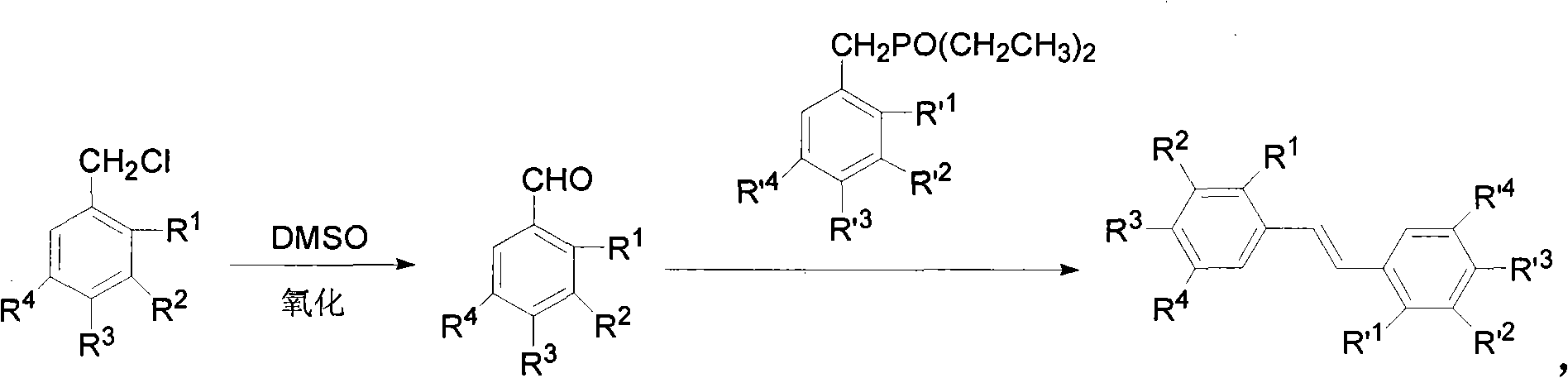
[0101] Example 1 A method for synthesizing 3,5-dimethoxy-4-isopropyl stilbene that is styrene modder by Kornblum oxidation reaction
[0102] The reaction scheme of present embodiment is as formula (II):
[0103]
[0104] Formula (II)
[0105] Its reaction process is a 1 →b 1 → c 1 , the step-by-step reaction is as follows:
[0106]
[0107] Utilize the method of Kornblum oxidation reaction to synthesize phenylene moder to carry out according to the following step sequence:
[0108] a 1 .Compound A 1 That is, the preparation of 3,5-dimethoxy-4-isopropylbenzaldehyde:
[0109] Take 10.0g (43.8mmol) of 3,5-dimethoxy-4-isopropylbenzyl chloride, 62.1mLDMSO, 11.0g NaHCO 3 Add it into a four-neck flask, stir, react at room temperature, and monitor by TLC. After the reaction is completed, add water, extract with ethyl acetate, wash with water, and remove the solvent by rotary evaporation to obtain product A 1 7.83g (37.6mmol). Yield 86%.
[0110] b 1 .Compound B 1 T...
Embodiment 2
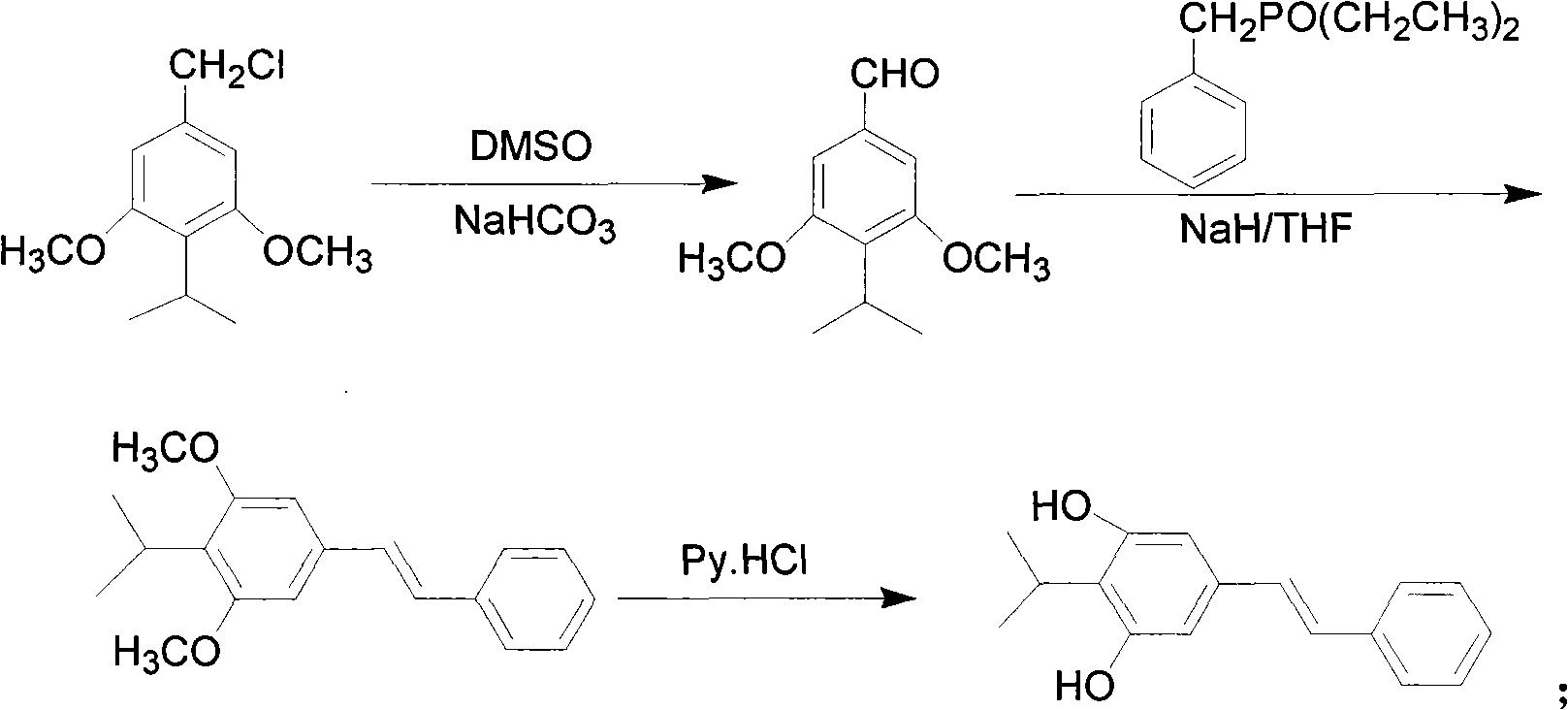
[0119] Example 2 The method of synthesizing 3,5-dimethoxy-4'-hydroxystilbene, namely pterostylbene by Kornblum oxidation method
[0120] The reaction scheme of present embodiment is as formula (III):
[0121]
[0122] Formula (III)
[0123] Its reaction process is a 2 →b 2 → c 2 , the step-by-step reaction is as follows:
[0124]
[0125] This method is carried out according to the following sequence of steps:
[0126] a 2 .Compound A 2 That is, the preparation of 3,5-dimethoxybenzaldehyde:
[0127] Take 10.0g (53.6mmol) of 3,5-dimethoxybenzyl chloride, 114.1mLDMSO, 27.0g NaHCO 3 Add it into a four-neck flask, stir, react at room temperature, and monitor by TLC. After the reaction is completed, add water, extract with ethyl acetate, wash with water, and remove the solvent by rotary evaporation to obtain product A 2 7.97 (48.0 mmol). Yield 88.5%.
[0128] b 2 .Compound B 2 That is, the preparation of 3,5-dimethoxy-4'-tert-butyldimethylsiloxy stilbene:
[01...
Embodiment 3
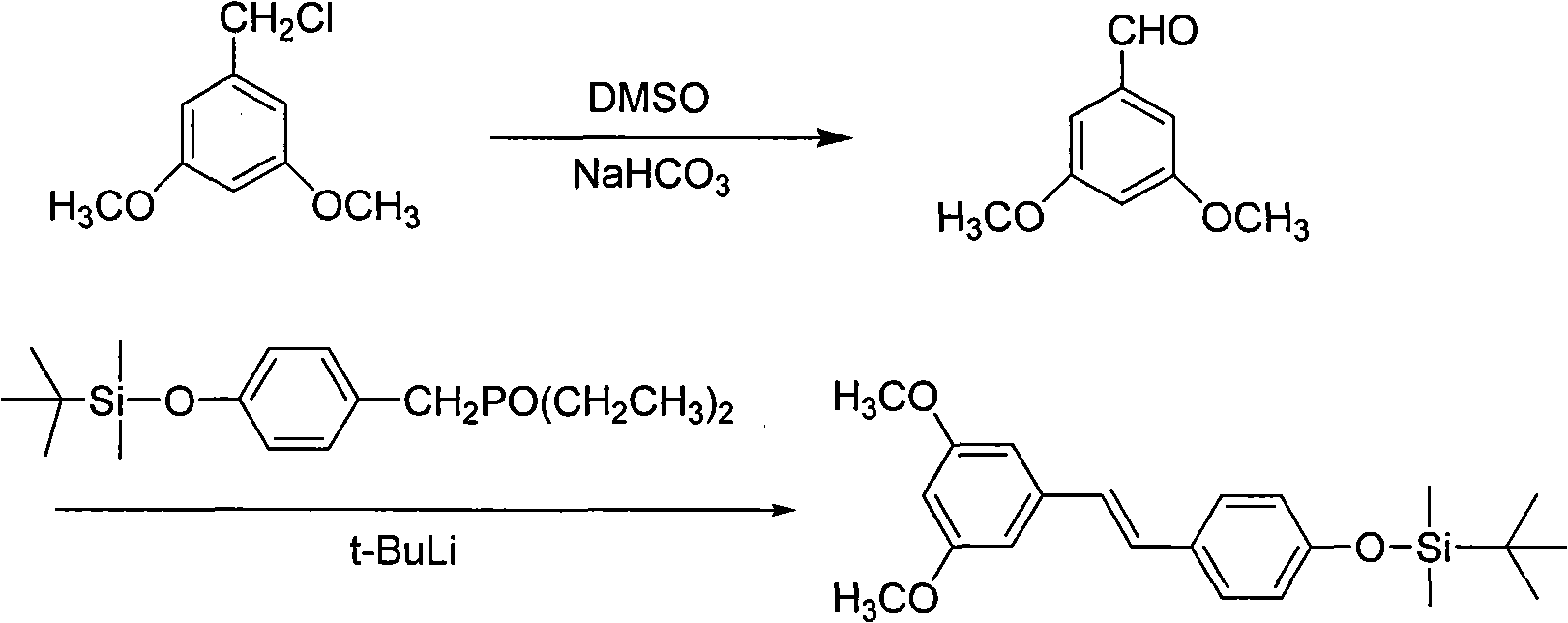
[0137] Example 3 Synthesis method 1 of the method for synthesizing 3,5,4'-trihydroxystilbene, that is, resveratrol by Kornblum oxidation reaction
[0138] The reaction scheme of present embodiment is as formula (IV):
[0139]
[0140]
[0141] Formula (IV)
[0142] Its reaction process is a3 →b 3 → c 3 , the step-by-step reaction is as follows:
[0143]
[0144] This method is carried out according to the following sequence of steps:
[0145] a 3 .Compound A 3 That is, the preparation of 3,5-dimethoxybenzaldehyde
[0146] Take 10.0g (53.6mmol) of 3,5-dimethoxybenzyl chloride, 38.0mLDMSO, 4.5gNaHCO 3 Add it into a four-neck flask, stir, react at room temperature, and monitor by TLC. After the reaction is completed, add water, extract with ethyl acetate, wash with water, and remove the solvent by rotary evaporation to obtain product A 3 7.80 g (47.0 mmol). Yield 87.6%.
[0147] b 3 .Compound B 3 That is, the preparation of 3,5,4'-trimethoxystilbene
[0148...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com