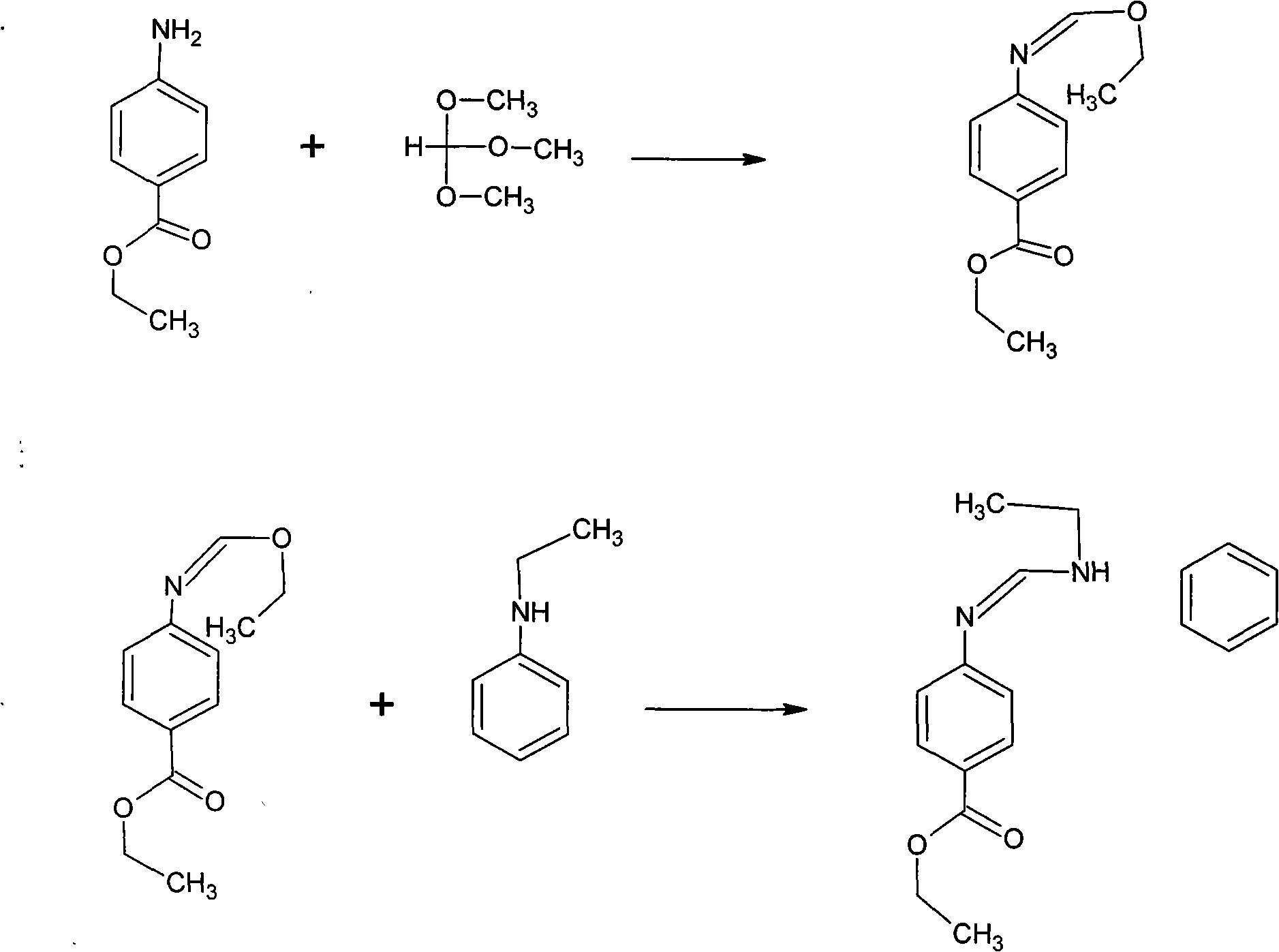
Preparation method of N-(4-ethoxycarbonylphenyl)-N'-ethyl-N'-phenylformamidine
A technology of ethoxycarbonyl phenyl and phenylformamidine, which is applied in the field of preparation of N--N'-ethyl-N'-phenylformamidine, and can solve the problems of limiting large-scale industrial production, color and purity. , separation and purification are cumbersome and other problems, to achieve the effect of reducing energy consumption and labor intensity, product color is good, saving labor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put 150 kilograms of ethyl p-aminobenzoate, 270 kilograms of triethyl orthoformate and 150 kilograms of N-ethylaniline into a 1000-liter reactor with a separation tower, start stirring and heat up. When the temperature rises to 100°C, ethanol is generated, and the temperature is raised slowly to control the temperature at the top of the tower to be less than 78°C. When the ethanol is received to 110 liters, the temperature of the kettle is about 110-140°C. Keep it warm for 3 hours to recover triethyl orthoformate For ester, N-ethylaniline is recovered by distillation under reduced pressure. The temperature is controlled at 160°C. After the reaction is completed, it is moved to a 500-liter distillation pot for distillation. After removing the fore distillation, collect the fraction at 222-228°C / 1mmHg to obtain 250 kg of distillate. Then add 250 kilograms of distillate into a 1000-liter reaction kettle with 500 kilograms of methyl alcohol and stir to freeze and crystalliz...
Embodiment 2
[0022] Put 150 kilograms of ethyl p-aminobenzoate, 350 kilograms of triethyl orthoformate and 170 kilograms of N-ethylaniline into a 1000-liter reactor with a separation tower, start stirring and heat up. When the temperature rises to 100°C, ethanol is generated, and the temperature is raised slowly to control the temperature at the top of the tower to be less than 78°C. When the ethanol is received to 120 liters, the temperature of the kettle is about 110-140°C. Keep it warm for 3 hours to recover triethyl orthoformate For ester, N-ethylaniline is recovered by distillation under reduced pressure. The temperature is controlled at 160°C. After the reaction is completed, it is moved to a 500-liter distillation pot for distillation. After the fore distillation was removed, the fraction at 222-228°C / 1mmHg was collected to obtain 258 kg of distillate. Add 258 kilograms of distillate to 510 kilograms of methyl alcohol and freeze and crystallize in a 1000-liter reactor with stirring....
Embodiment 3
[0024] Put 150 kilograms of ethyl p-aminobenzoate, 270 kilograms of trimethyl orthoformate and 160 kilograms of N-ethylaniline into a 1000-liter reactor with a separation tower, start stirring and heat up. When the temperature rises to 80°C, methanol is produced, and the temperature is raised slowly to control the temperature at the top of the tower to be less than 68°C. When the methanol is received to 110 liters, the temperature of the kettle is about 110-140°C. Keep it warm for 3 hours to recover trimethyl orthoformate For ester, N-ethylaniline is recovered by distillation under reduced pressure. The temperature is controlled at 160°C. After the reaction is completed, it is moved to a 500-liter distillation pot for distillation. After the fore distillation was removed, the fraction at 222-228°C / 1mmHg was collected to obtain 256 kg of distillate. Add 256 kilograms of distillate to 500 kilograms of methyl alcohol and freeze crystallization in a 1000-liter reactor with stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com