Cefpiramide sodium hydrate and preparation method and application thereof
A technology of cefpiramide sodium and hydrate, applied in the field of medicine, can solve the problems of reporting the preparation method and use of cefpiramide sodium crystal hydrate, and achieve the effects of improving operability, good sliding property, and being convenient for storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
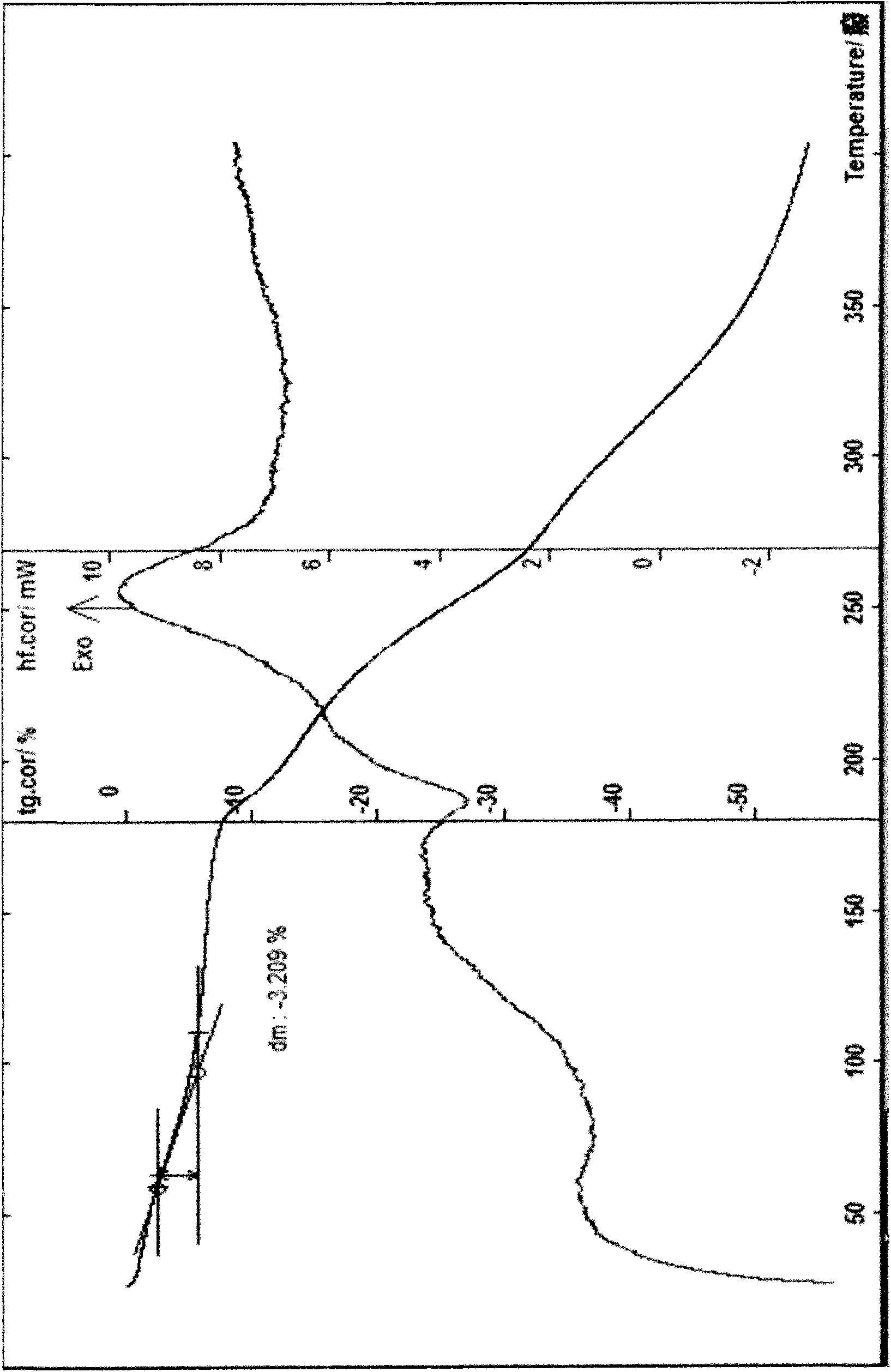
[0037] Example 1 Preparation of Cefpiramide Sodium Monohydrate Add 10 g of cefpiramide, 20 ml of water, and 30 ml of methanol in a reaction flask, stir, add 2.4 ml of triethylamine dropwise with stirring at 5° C., stir for 30 minutes, add 0.1 ml of activated carbon g, stirred for 30 minutes, filtered with suction, washed with water, filtered with suction, added dropwise an ethanol solution of 25% sodium isooctanoate (2.8g) in the filtrate at 5°C, stirred, slowly added 250ml of acetone and 250ml of ethanol dropwise, below -6°C Put it aside to allow the solid to be fully separated, filter with suction, wash with a small amount of chloroform for 3 times, and filter with suction, dissolve the obtained solid with a small amount of water, add 300ml of acetone and 300ml of isopropanol for recrystallization, and place it below -6°C to make the solid fully Precipitate, filter with suction, wash with a small amount of chloroform for 3 times, filter with suction, and dry the obtained soli...
Embodiment 2
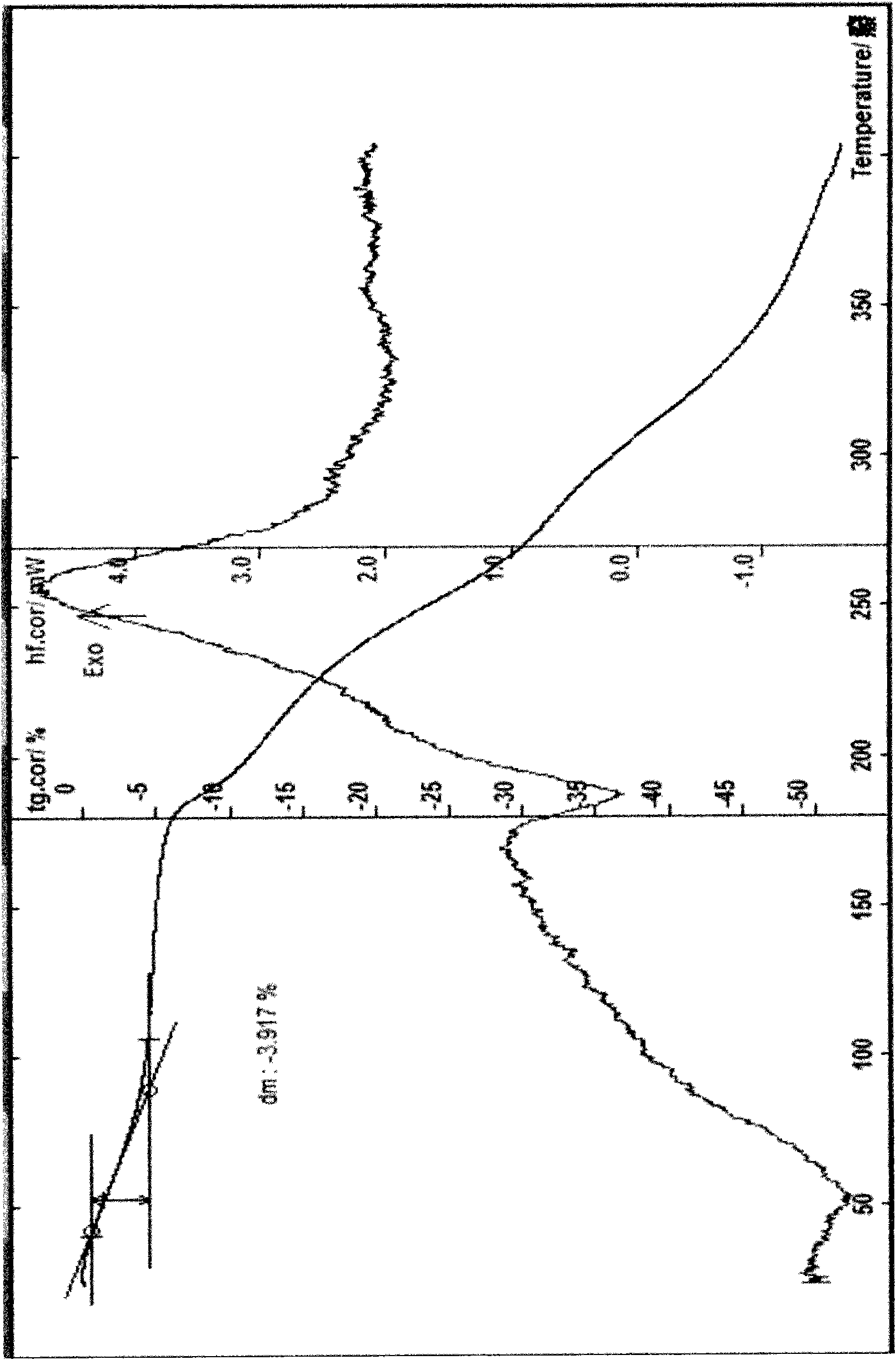
[0038]Example 2 Preparation of Cefpiramide Sodium Monohydrate Add 20 g of cefpiramide sodium and 80 ml of water into the reaction flask, stir to form a suspension, add saturated aqueous sodium carbonate solution dropwise at 5°C to make the pH to about 6.8, and stir For 30 minutes, add 0.5g of activated carbon, stir for 30 minutes, filter with suction, wash with water, filter with suction, slowly add 250ml of acetone and 300ml of acetonitrile dropwise, place below -4°C to fully separate out the solid, filter with suction, wash with acetone for 3 times, and filter with suction , the resulting solid was just dissolved with a small amount of water, recrystallized with 300ml of acetone, 300ml of acetonitrile, and 10ml of chloroform, and placed overnight below -10°C to allow the crystals to be fully separated, filtered with suction, washed with a small amount of acetone for 3 times, filtered with suction, and kept at 52°C Vacuum-dried for about 6 hours to obtain 13.4 g of off-white c...
Embodiment 3
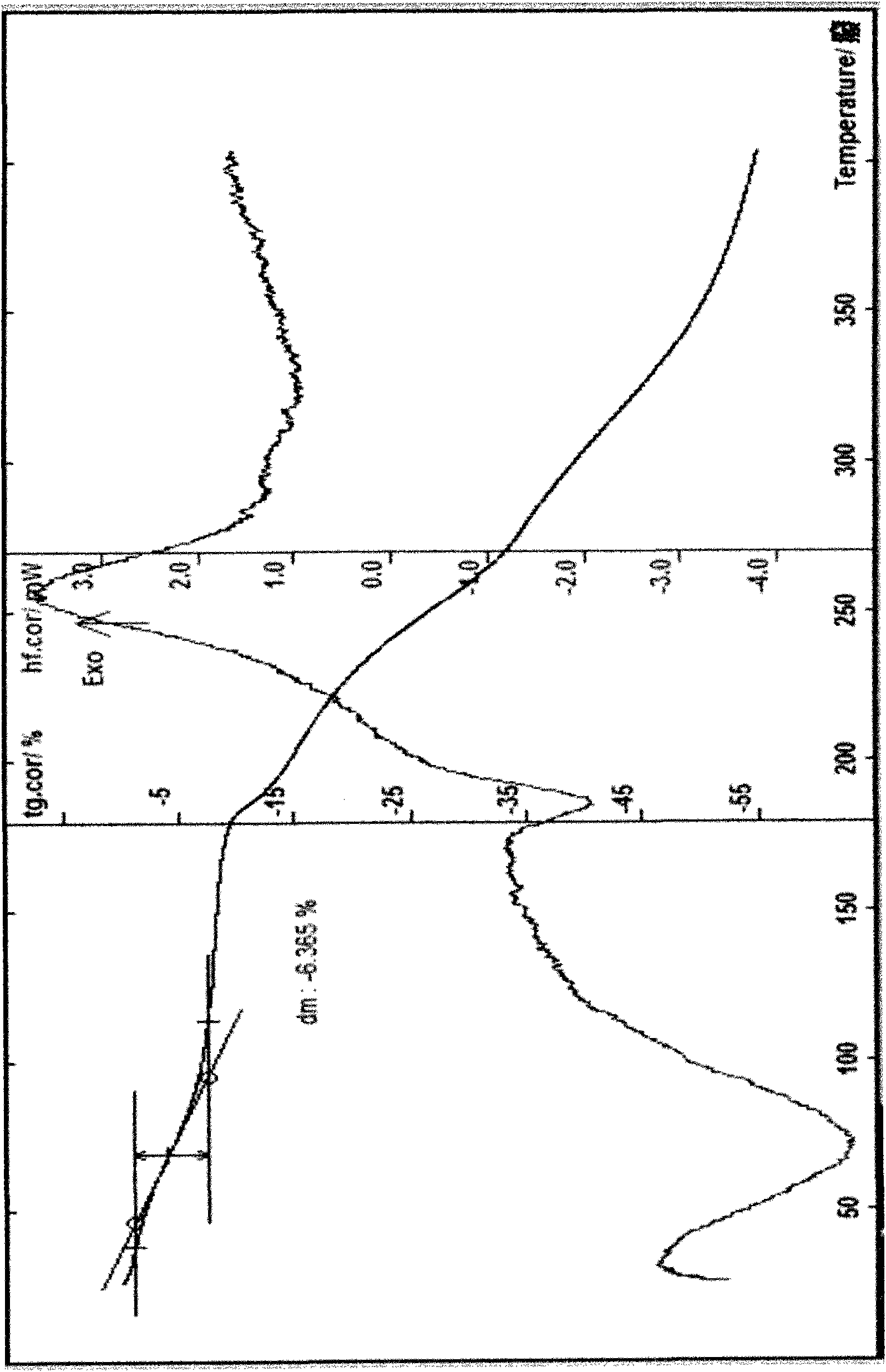
[0039] Example 3 Preparation of Cefpiramide Sodium 1.5 Hydrate Add 20 g of cefpiramide and 80 ml of water into the reaction flask, stir to form a suspension, add dropwise a saturated aqueous solution of sodium bicarbonate under stirring at 5°C to bring the pH to about 6.8, and stir 30 minutes, add 0.3g of activated carbon, stir for 30 minutes, filter with suction, wash with water, filter with suction, slowly add 350ml of acetone and 250ml of ethanol, place below -5°C, let the solid fully separate out, filter with suction, wash with ethanol 3 times, and filter with suction , the obtained solid was just dissolved with a small amount of water, added 300ml of ethanol, 300ml of acetonitrile, and 80ml of isopropyl ether as a crystallization solvent for recrystallization, and placed overnight below -10°C to fully separate out the crystals, suction filtered, and vacuum-dried at 45°C at 0.06MPa About 5 hours, 10.6g of off-white crystals were obtained, melting point: discoloration at 170...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com