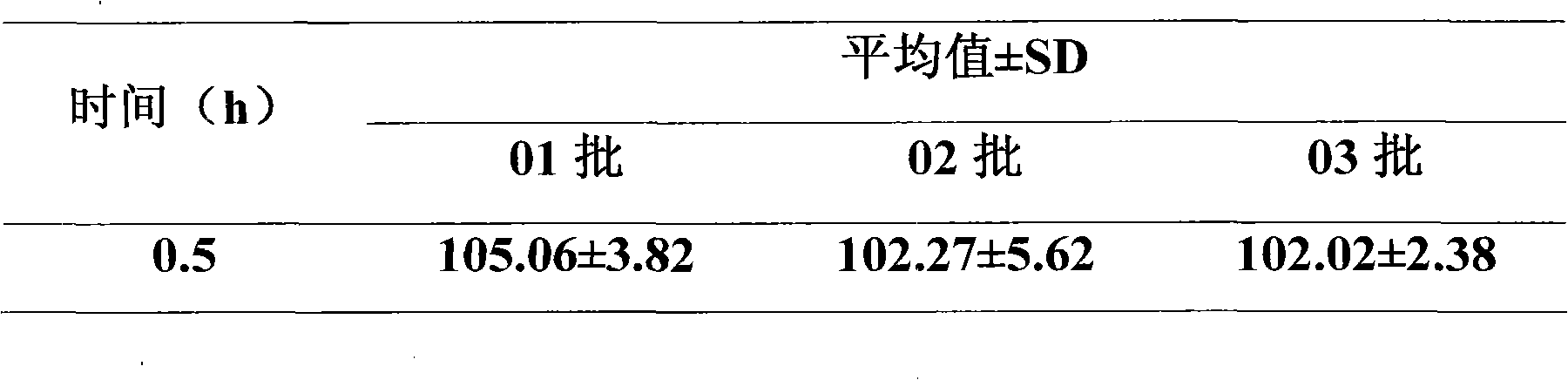
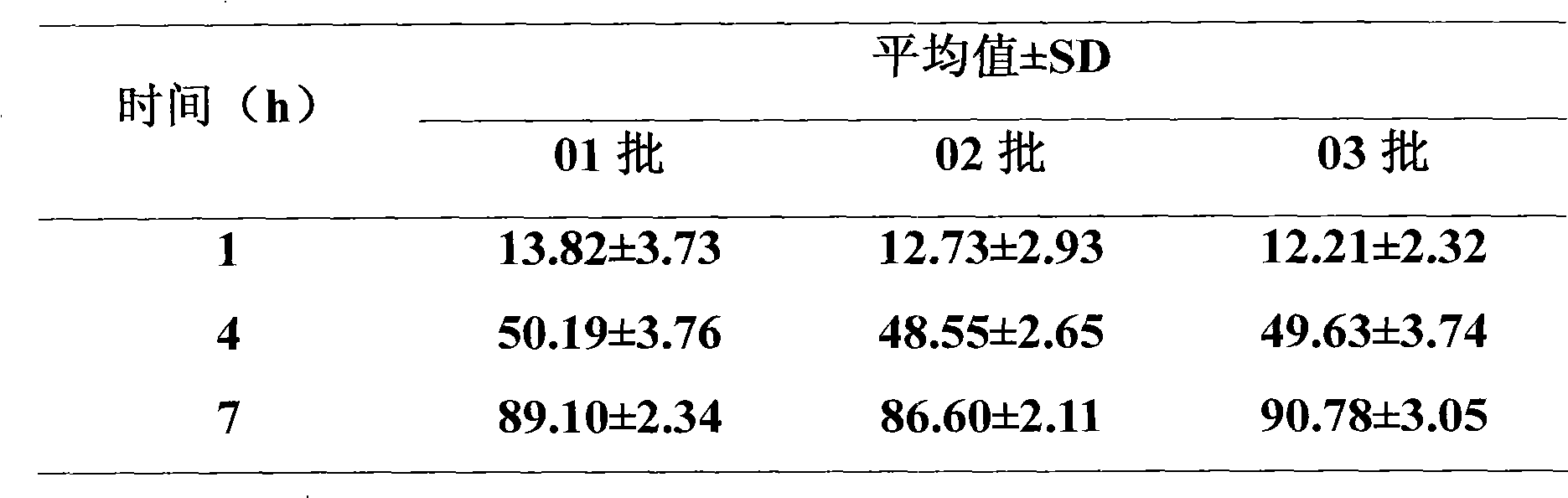
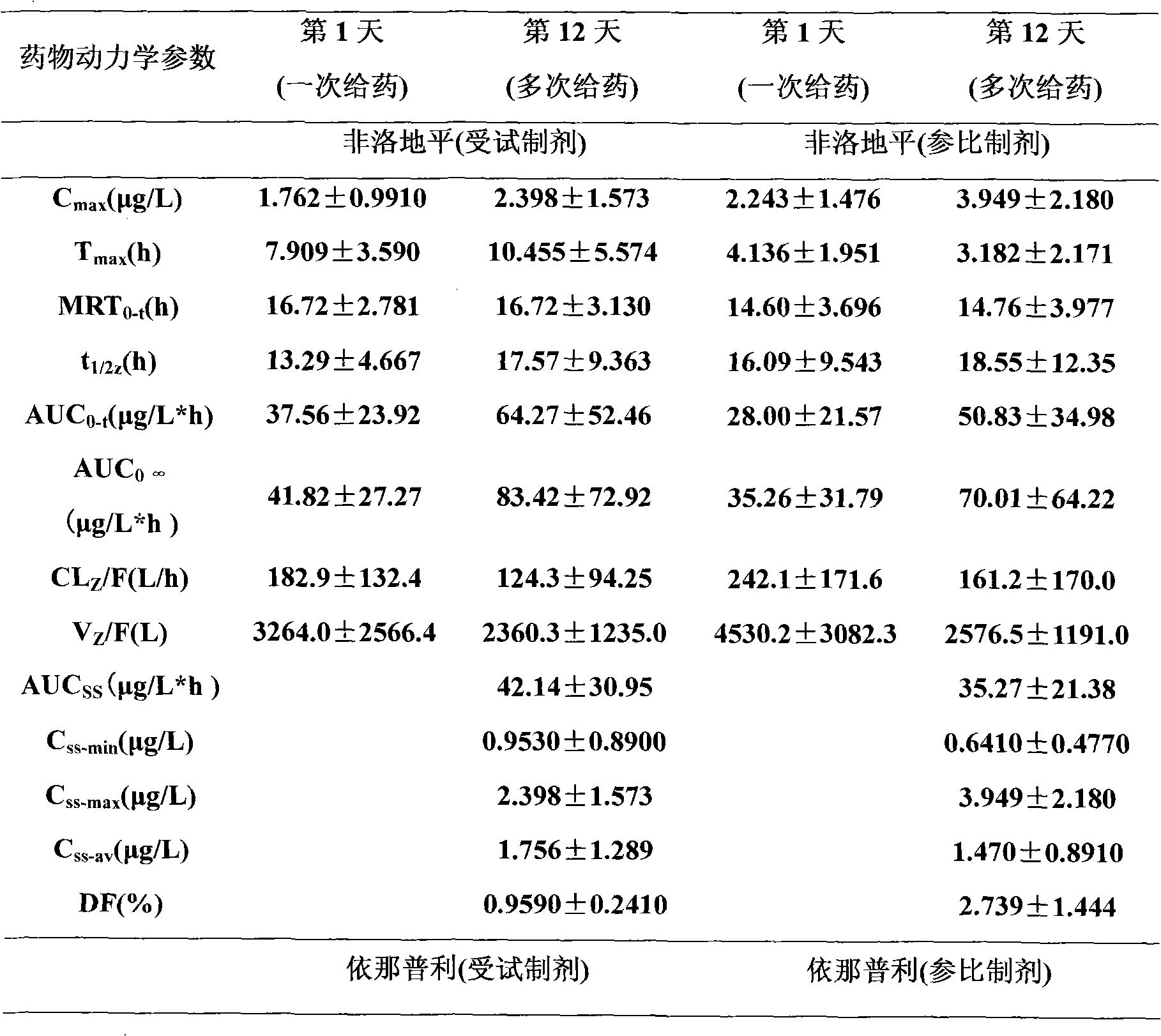
Medicament composition comprising Enalapril quick-releasing part and felodipine slow-releasing part
A technology of enalapril and sustained-release part, which is applied in the directions of drug combination, drug delivery, medical preparations containing active ingredients, etc. Insignificant and other problems, to achieve the effect of strong patient compliance, less adverse reactions, and rapid stabilization of blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Enalapril and felodipine sustained-release tablets (1000 formulations)
[0042] Chip:
[0043] Felodipine 2.5g
[0044] HPMC65g
[0045] Lactose 200g
[0047] 95% ethanol solution 180g
[0048] Immediate release coating layer prescription (for preparing coating solution):
[0049] Enalapril Maleate 2.5g
[0050] Opadry 50g
[0051] Purified water 1000g
[0052] Prescription of protective layer (prepared as coating solution):
[0053] Opadry 50g
[0054] Purified water 1000g
[0055] Preparation:
[0056] Felodipine and HPMC were passed through a 100-mesh sieve, and lactose and talcum powder were passed through a 80-mesh sieve. Raw materials (i.e. the drug felodipine) / excipients are mixed evenly, and then the soft material is made with an appropriate amount (that is, the amount given above) of ethanol solution, granulated with a 16-mesh sieve, dried, granulated with a 18-mesh sieve, added with talcum powder and mixed even...
Embodiment 2
[0057] Embodiment 2: Enalapril and felodipine sustained-release tablets (1000 formulations)
[0058] Chip:
[0059] Felodipine 5g
[0060] HPMC 50g
[0061] Lactose 100g
[0062] Microcrystalline Cellulose 55g
[0064] Hydrogenated Castor Oil 5g
[0065] povidone k 30 6g
[0066] 95% ethanol solution 120g
[0067] Purified water 15g
[0068] Immediate release coating formulation:
[0069] Enalapril Maleate 5g
[0070] Opadry 10g
[0071] Purified water 200g
[0072] Protective Layer Prescription:
[0073] Opadry 10g
[0074] Purified water 200g
[0075] Preparation:
[0076] Felodipine and HPMC were passed through a 100-mesh sieve, and microcrystalline cellulose, lactose, povidone k30, and magnesium stearate were passed through a 80-mesh sieve. After the raw / excipient materials are mixed evenly, use an appropriate amount (that is, the amount given above) to make soft materials with ethanol solution, granulate with a 16-mesh sieve,...
Embodiment 3
[0077] Embodiment 3: Enalapril and felodipine sustained-release tablets (1000 formulations)
[0078] Chip:
[0079] Felodipine 10g
[0080] HPMC 80g
[0081] Lactose 60g
[0082] Microcrystalline Cellulose 70g
[0083] Silica 2g
[0085] 95% ethanol solution 150g
[0086] Purified water 40g
[0087] Immediate release coating formulation:
[0088] Enalapril Maleate 10g
[0089] Opadry 20g
[0090] Purified water 400g
[0091] Protective Layer Prescription:
[0092] Opadry 5g
[0093] Purified water 200g
[0094] Preparation:
[0095] Felodipine and HPMC were passed through a 100-mesh sieve, and microcrystalline cellulose, lactose, silicon dioxide, and magnesium stearate were passed through a 80-mesh sieve. After the raw materials / auxiliary materials are evenly mixed, use an appropriate amount of ethanol solution to make a soft material, granulate with a 16-mesh sieve, dry, granulate with a 18-mesh sieve, add magnesium stearate and sil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com