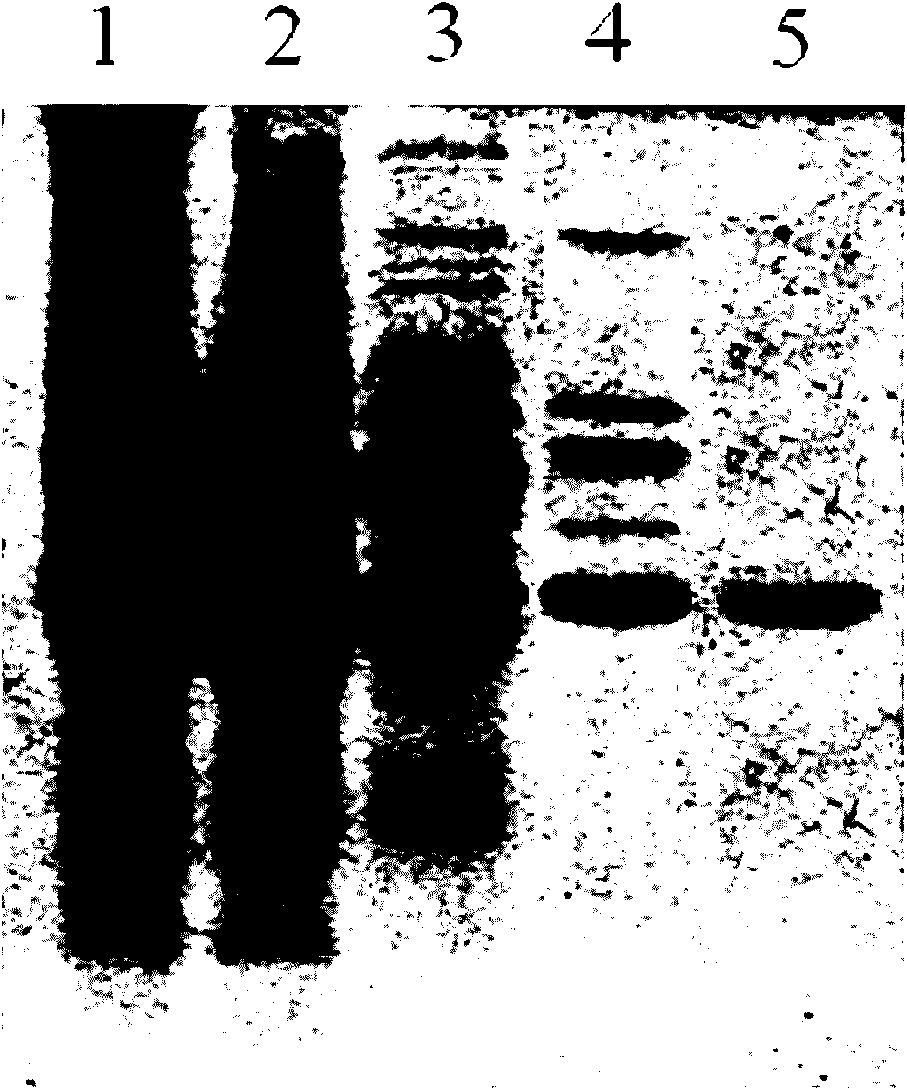Process for producing D(-)-tartaric acid by biotransformation
A biotransformation and tartaric acid technology, applied in the field of bioengineering, achieves the effects of good performance, reduced production costs, and reduced equipment volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
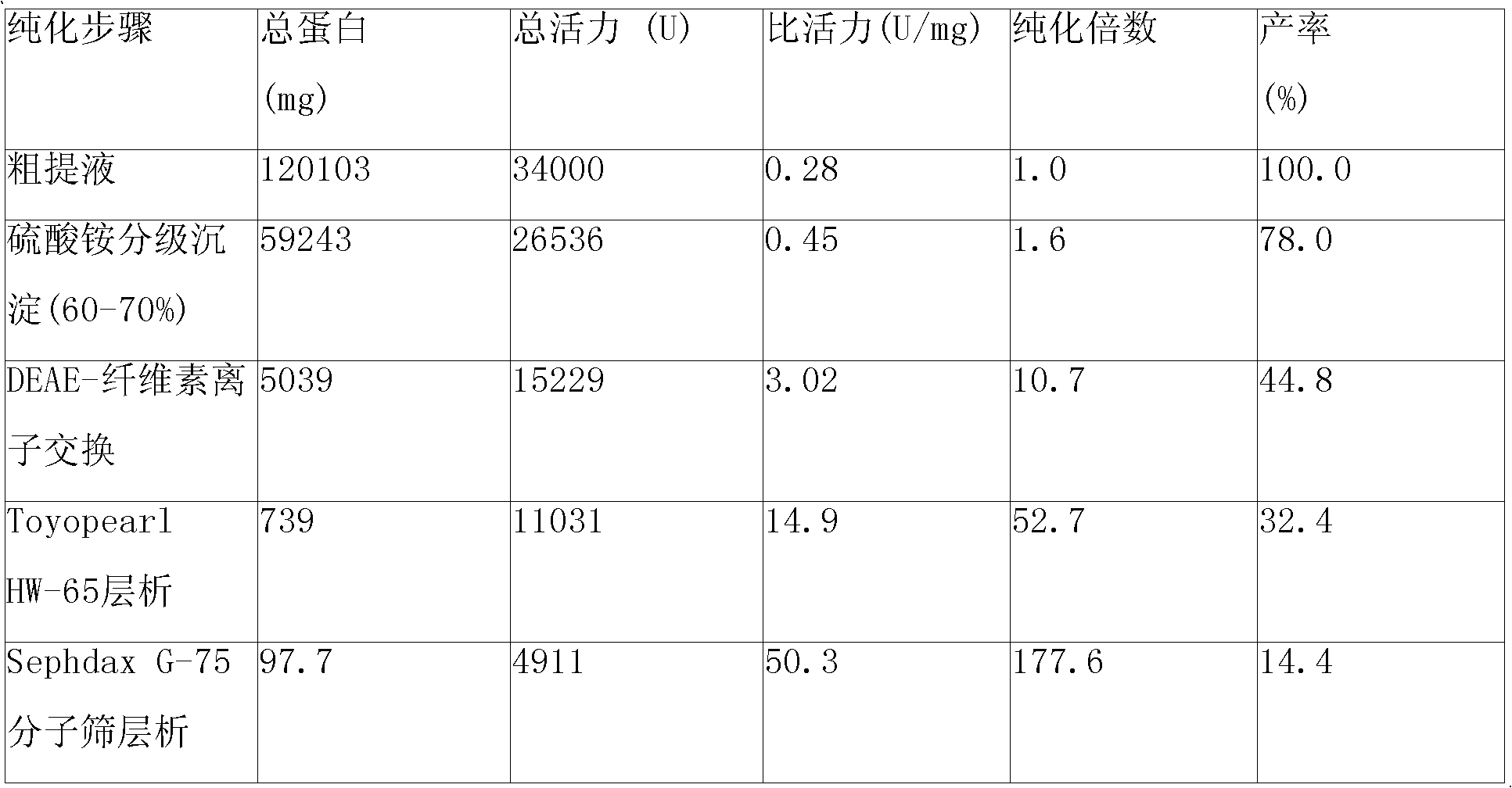
[0011] 1. Purification of cis-epoxysuccinate hydrolase
[0012] Use a strain (Bordetella sp.Strain 1-3) producing epoxysuccinate hydrolase, ferment in a 10-liter fermenter at 30°C for 30 hours, then centrifuge at 8000rpm for 20min to collect the bacteria, and use 50mmol / l pH 7.5 Cells were washed once with phosphate buffered saline. The formula of the fermentation broth is: yeast extract 7.8g / l, cis-epoxysuccinic acid 9.8g / l, KH 2 PO 4 1.12g / l, K 2 HPO 4 ·3H 2 O 3g / l, MgSO 4 ·7H 2 O 0.625g / l, trace element solution 12.5ml / l, pH 7.0. Afterwards, the obtained cells were suspended in 2 times the volume of 50 mmol / l pH 7.5 phosphate buffer, the cells were broken with a high-pressure homogenizer, and the cell wall fragments were removed by centrifugation at 8000 rpm for 20 min to obtain cis Crude extract of epoxysuccinate hydrolase.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com