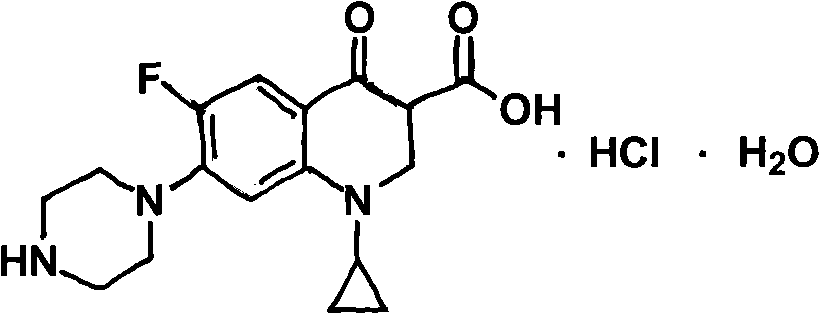
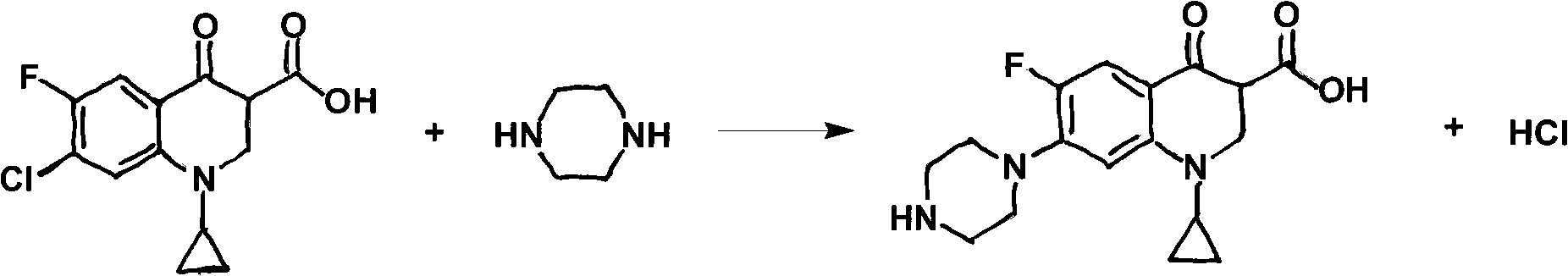
Preparation method of ciprofloxacin hydrochloride
A technology of ciprofloxacin hydrochloride and hydrochloric acid, which is applied in organic chemistry, anti-infective drugs, and drug combinations, etc., can solve problems affecting product quality and appearance, difficult biodegradation, and large environmental pollution, and achieve improvement in appearance and quality indicators , easy biodegradation, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Diphenhydramine
[0031] Put 30g of cyclopropanecarboxylic acid (0.1065mol), 50g of piperazine (0.5805mol), and 150ml of 2-pyrrolidone into a 250ml clean three-necked flask. Stir evenly, heat up to 70-80°C, and keep the temperature for about 3 hours. Sampling liquid phase monitoring, carboxylic acid residues are controlled below 1.5%. After the heat preservation reaction is completed, the temperature is lowered to 28±2°C. After cooling down, let stand for 3 hours, filter with suction, rinse with 30ml×3 medicinal alcohol to obtain 55-65g wet crude product.
[0032] (2) into salt
[0033] Put the above crude wet product into a 250ml three-necked bottle, add 150g of water, stir and heat up to 85°C, add hydrochloric acid dropwise until the system is basically dissolved, add 1.0g of activated carbon, stir for 30 minutes, decolorize, filter while hot to another 250ml clean In a three-necked flask, 0.8 g of acid-insoluble matter was filtered, and hydrochloric acid was ...
Embodiment 2
[0035] The difference from Example 1 is that 2-pyrrolidone is used as solvent, and K is added when reducing piper 2 CO 3 It is an acid-binding agent, and the molar yield of the final product is 77.8%.
Embodiment 3
[0037] (1) Diphenhydramine
[0038] Put 30g of cyclopropanecarboxylic acid (0.1065mol), 40g of piperazine (0.4644mol), and 120ml of N-ethylpyrrolidone into a 250ml clean three-necked flask. Stir evenly, heat up to 110-115°C, and keep the temperature for about 10 hours. Sampling liquid phase monitoring, carboxylic acid residues are controlled below 1.5%. After the heat preservation reaction is completed, the temperature is lowered to 20±2°C. After cooling down, let stand for 3 hours, filter with suction, rinse with 30ml×3 medicinal alcohol to obtain 65-70g wet crude product.
[0039] (2) into salt
[0040] Put the above crude wet product into a 250ml three-neck bottle, add 150g of water, stir and heat up to 85°C, add hydrochloric acid dropwise until the system is basically dissolved, add 1.5g of activated carbon, stir for 30 minutes, decolorize, filter while hot to another 250ml clean In a three-necked flask, 0.9 g of acid-insoluble matter was filtered, and hydrochloric aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com