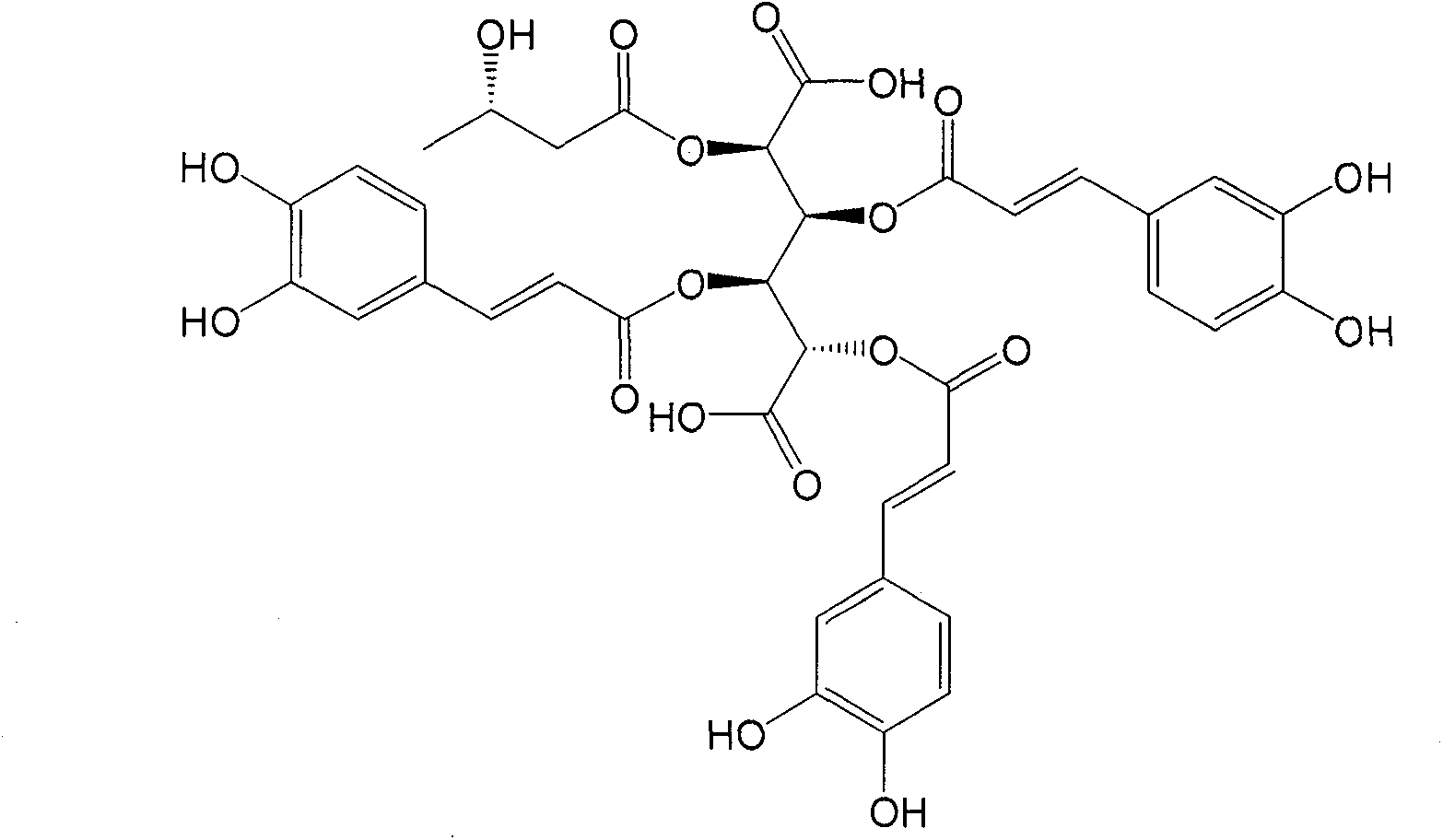
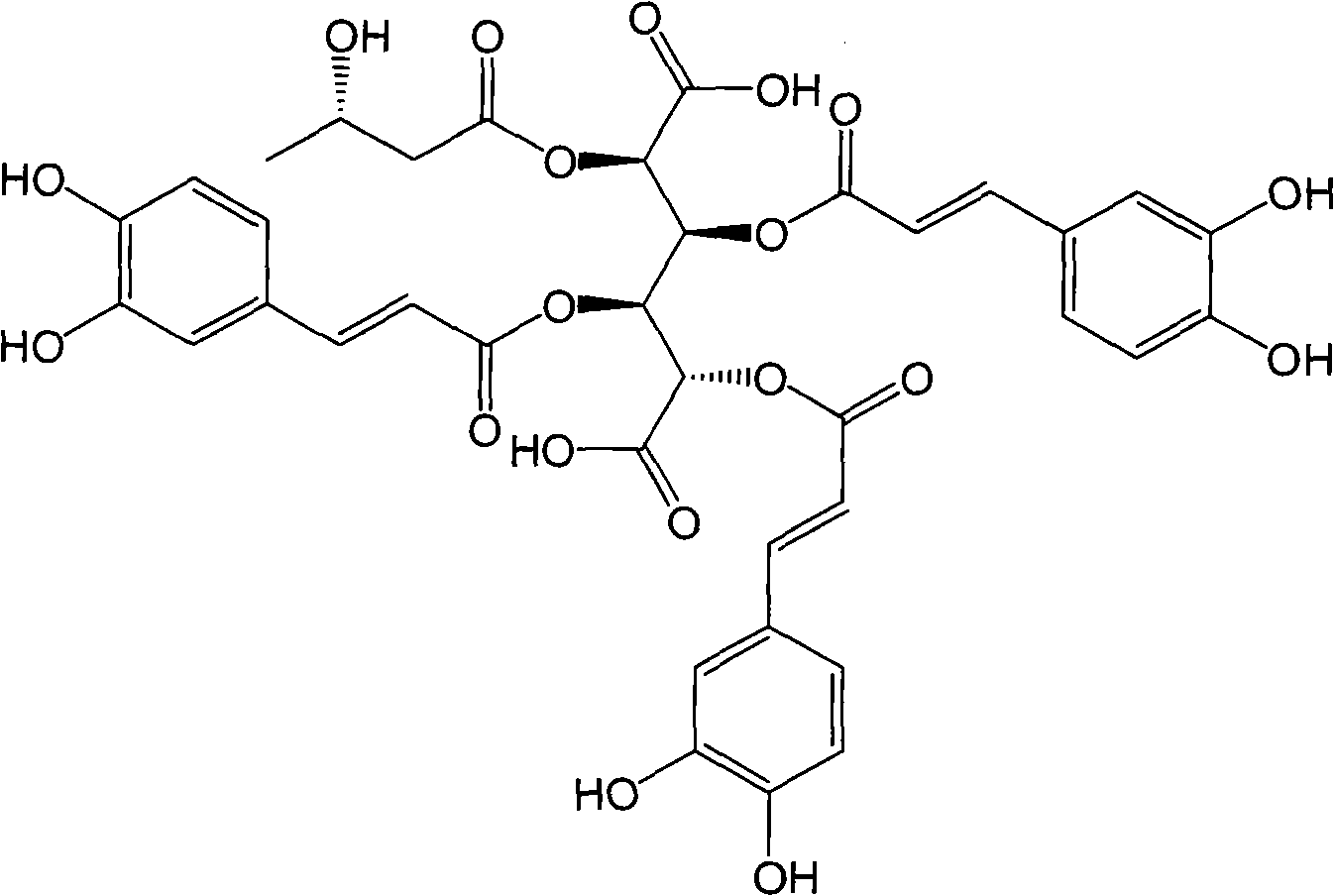
Extract of leontopodic acid plant and application of active ingredients thereof in treating hepatitis
A technology of plant extracts and active ingredients, applied in the field of preparation of drugs for the treatment of hepatitis B, can solve the problem that there are no anti-hepatitis drugs of edelweisin compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
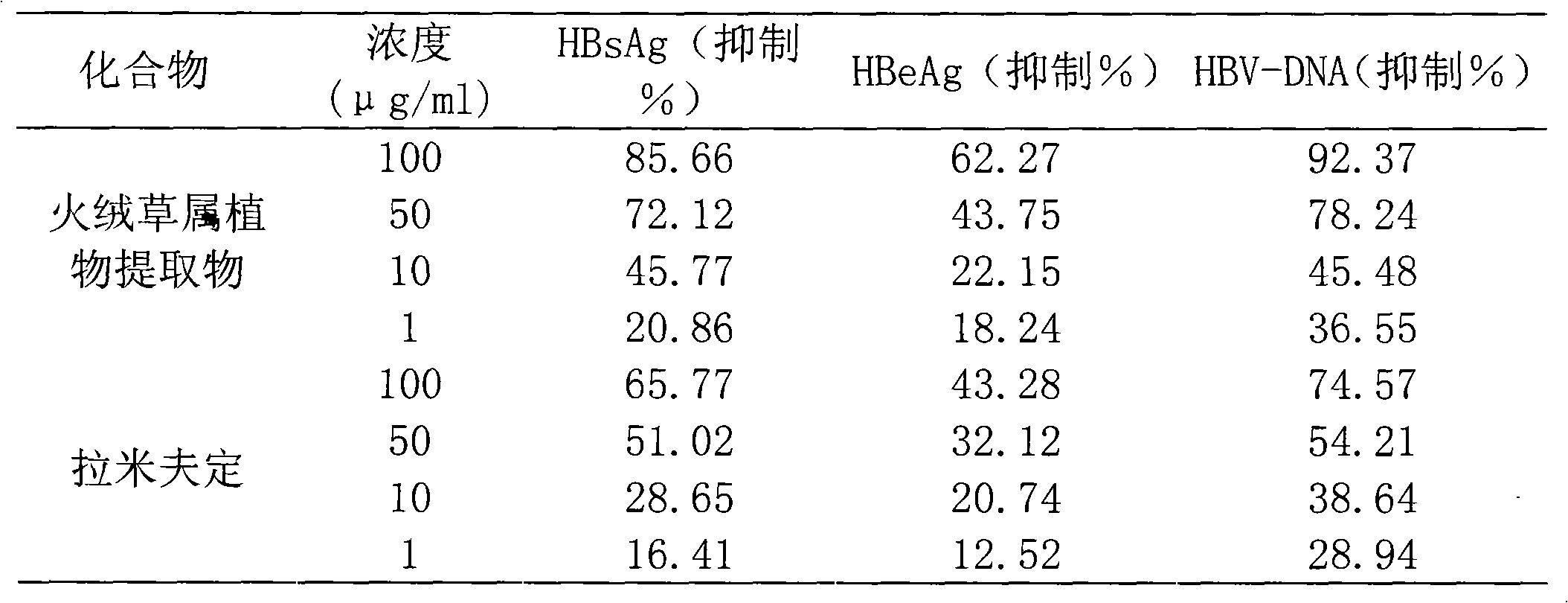
[0020] Example 1: Inhibition of Edelweiss plant extracts on HepG2.2.15 cells secreting hepatitis B virus surface antigen (HBsAg), hepatitis B virus E antigen (HBeAg) and hepatitis B virus DNA (HBV-DNA).
[0021] Determination of the inhibitory effect of the sample on the growth of HepG2.2.15 cells: the cells were digested with trypsin-EDTA and diluted to 1×10 5 / mL, added to a 96-well cell culture plate, 100uL per well, placed in CO 2cultured in an incubator. After 24 hours of inoculation, discard the medium, add the sample diluted with the medium, 200ul per well, add 3 wells for each concentration, add 5mg / ml MTT to the cell culture wells after 72 hours of culture, 10ul per well, and place at 37°C After incubation for 3 hours, 150ul of DMSO was added to completely dissolve the formazan, and a microplate reader was used for colorimetry at a wavelength of 570nm. Calculate the median toxic concentration of the sample on the growth of HepG2.2.15 cells.
[0022] Determination o...
Embodiment 2
[0027] Example 2 Effect of Edelweiss plant extract on D-GalN-induced liver cell damage
[0028] Human normal liver cell line HL-7702, placed at 37°C in CO 2 Culture in an incubator; after the hepatocytes adhered to the wall, replace the culture medium, add 200mmol / l D-GalN, and act for 8h; then discard the culture medium, and add 200μl of different concentrations of the test drug solution, each concentration was set at 6 Repeat wells, set solvent and positive control at the same time; continue to culture for 48h, add 10μl MTT (5mg / ml) to each well, act for 4h, aspirate and discard the supernatant, add 150μl DMSO, shake and mix well; The instrument measures the A value.
[0029] Protection evaluation: calculation of protection rate (%) and proliferation index.
[0030]
[0031] Note 2: Proliferation index = sample group (A) / injury group (A)
[0032] Table 2 Effects of Edelweiss plant extracts on human normal liver cell damage induced by D-GalN
[0033]
[0034] The re...
Embodiment 3
[0035] Example 3 Protective effect of edelweiss plant extract on D-GalN-induced acute liver injury model in mice
[0036] Materials: 20-25g male ICR mice were randomly divided into groups according to body weight, 10 mice in each group. A normal control group, an injury control group, a positive control group and high, medium and low dose groups of the test drug were set up. Animals in the drug group were given prophylactic administration once a day for 7 consecutive days. The normal control group and the injury control group were given an equal volume of solvent, and the positive control group was given 50 mg / kg of silymarin. One hour after the last administration, animals in each group except the normal group were injected with 650 mg / kg galactosamine (D-GalN) intraperitoneally to cause toxicity. 24 hours after the last administration, the serum was separated to measure aspartate aminotransferase (AST) and alanine aminotransferase (ALT). At the same time, liver tissue was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com