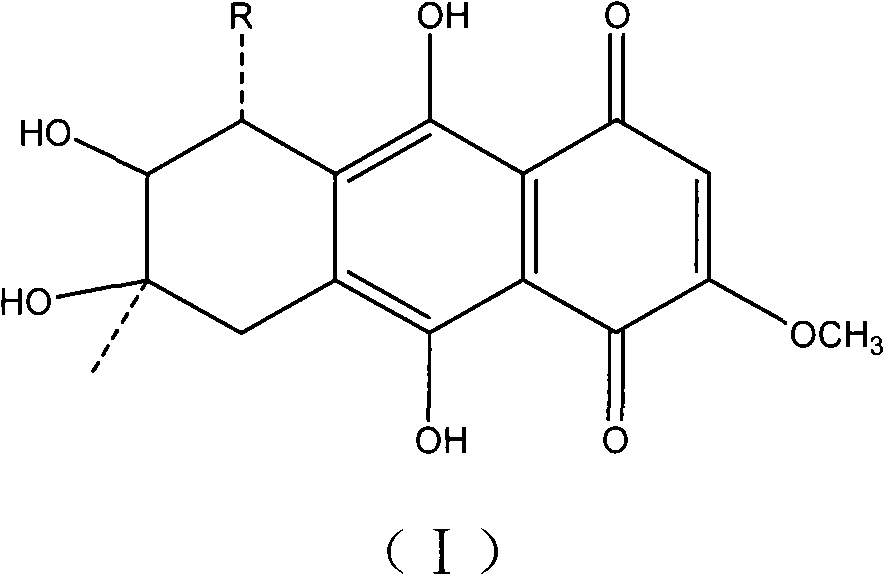
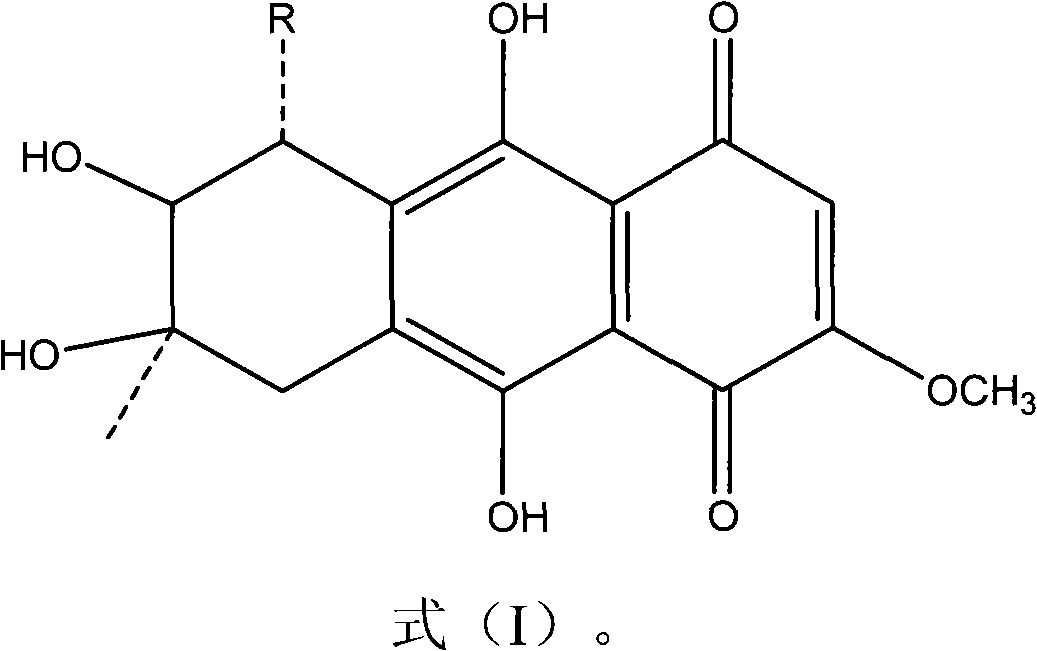
Application of quinine compound in preparing anti-tubercle bacillus drugs
A technology of anti-tuberculosis bacteria and compounds, applied in the direction of antibacterial drugs, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problems of non-standard treatment and management of tuberculosis patients, difficulties in tuberculosis prevention and control, and achieve the goal of expanding types Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of Example 1 1403B and 1403C
[0037] The quinone compounds 1403B and 1403C of the present invention can be extracted and separated from the fermentation broth of the marine fungus Halorosellinia sp.1403, and the specific steps of the preparation method are as follows:
[0038] (1) Seed culture of the fungus Halorosellinia sp.1403 CCTCC NO: M 201018: medium by weight: glucose 0.5-1.5, yeast extract 0.05-0.15, peptone 0.1-0.3, agar 1-1.5, sodium chloride 3 -5, water 100, make a test tube slope, pick the strains into the slope, and culture at 30-35°C for 5-7 days;
[0039] (2) Fermentation culture of fungus Halorosellinia sp.1403 CCTCC NO: M 201018: Fermentation medium by weight ratio: glucose 5-15, yeast extract 1-4, peptone 0.5-4, sodium chloride 3-5, water 100, picking the cultured strains in the slant into the fermentation medium, and standing at room temperature 25-35°C for 1-2 months;
[0040] (3) filtering the above-mentioned cultivated fermented liqu...
Embodiment 2
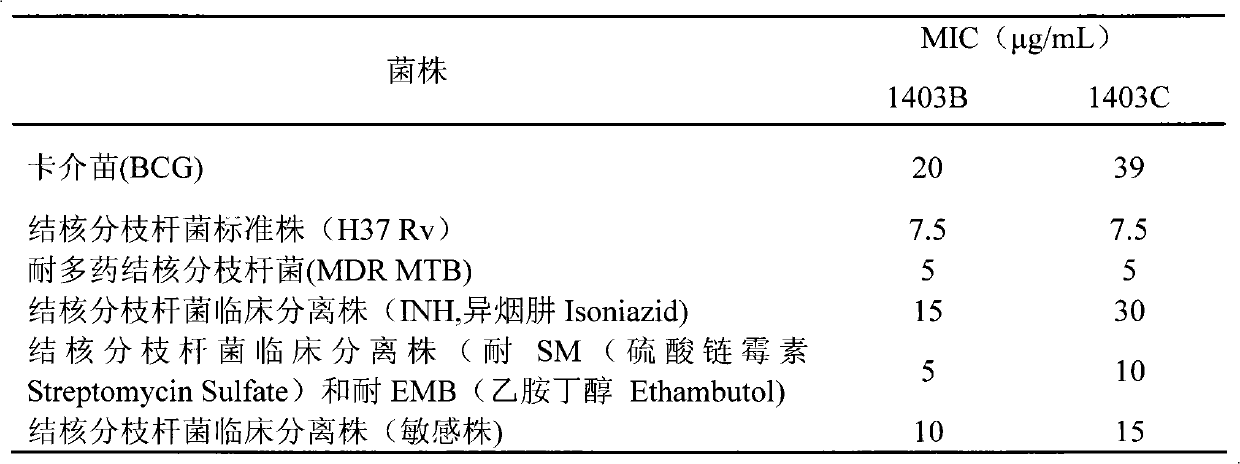
[0046] Example 2 Determination of Absolute Concentration of 1403B and 1403C Anti-Bacillus Calmette-Guerin (BCG) by Solid Medium Dilution Method
[0047] Scrape the BCG culture from the inclined surface, add it to 3ml Middlebrook7H9 broth medium, add a small amount of glass beads, tighten the test tube cap, vibrate vigorously on the vortex shaker, and compare it with the standard McFarland turbidimetric tube (MacFarland No. 1) Turbidity, that is, to prepare a 1 mg / ml bacillus Calmette-Guerin (BCG) bacterial suspension.
[0048] Make 1403B and 1403C into high-concentration stock solution with DMSO, dilute the stock solution to the required concentration with 5% Tween-80 sterile ultrapure water, add the diluted 1403B and 1403C to 4ml Middlebrook according to the required dose 7H11 agar medium (the medium has been sterilized by high-pressure steam at 121°C for 15 minutes, cooled to 50-55°C), mixed evenly to make 1403B and 1403C, the concentrations are 60μg / mL, 40μg / mL, 30μg / mL , ...
Embodiment 3
[0051] Example 3 Determination of the Absolute Concentration of 1403B and 1403C Anti-tuberculosis Mycobacterium Standard Strain H37Rv by Solid Medium Dilution
[0052] Scrape the culture of Mycobacterium tuberculosis standard strain H37Rv from the slant, add it to 3ml Middlebrook 7H9 broth medium, add a small amount of glass beads, tighten the test tube cap, vibrate vigorously on a vortex shaker, grind with standard McFarland The turbidimetric tube (MacFarland No.1) was used for turbidity, that is, a 1 mg / ml H37Rv strain suspension was prepared.
[0053] Make 1403B and 1403C into high-concentration stock solution with DMSO, dilute the stock solution to the required concentration with 5% Tween-80 sterile ultrapure water, add the diluted 1403B and 1403C to 4ml Middlebrook according to the required dose 7H11 agar medium (the medium has been sterilized by high-pressure steam at 121°C for 15 minutes, and cooled to 50-55°C), mix well to make 60μg / mL, 40μg / mL, 30μg / mL of 1403B and 14...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com