Polyethyleneimine-chitosan-octadecanoic acid grafting, preparation and application
A polyethylenimine and chitosan technology, which is applied in the directions of drug combinations, genetic material components, and inactive medical preparations, can solve the problems of low transfection efficiency, low surface-to-surface efficiency, etc. Buffering capacity, improving hydrophobicity, overcoming toxicity issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
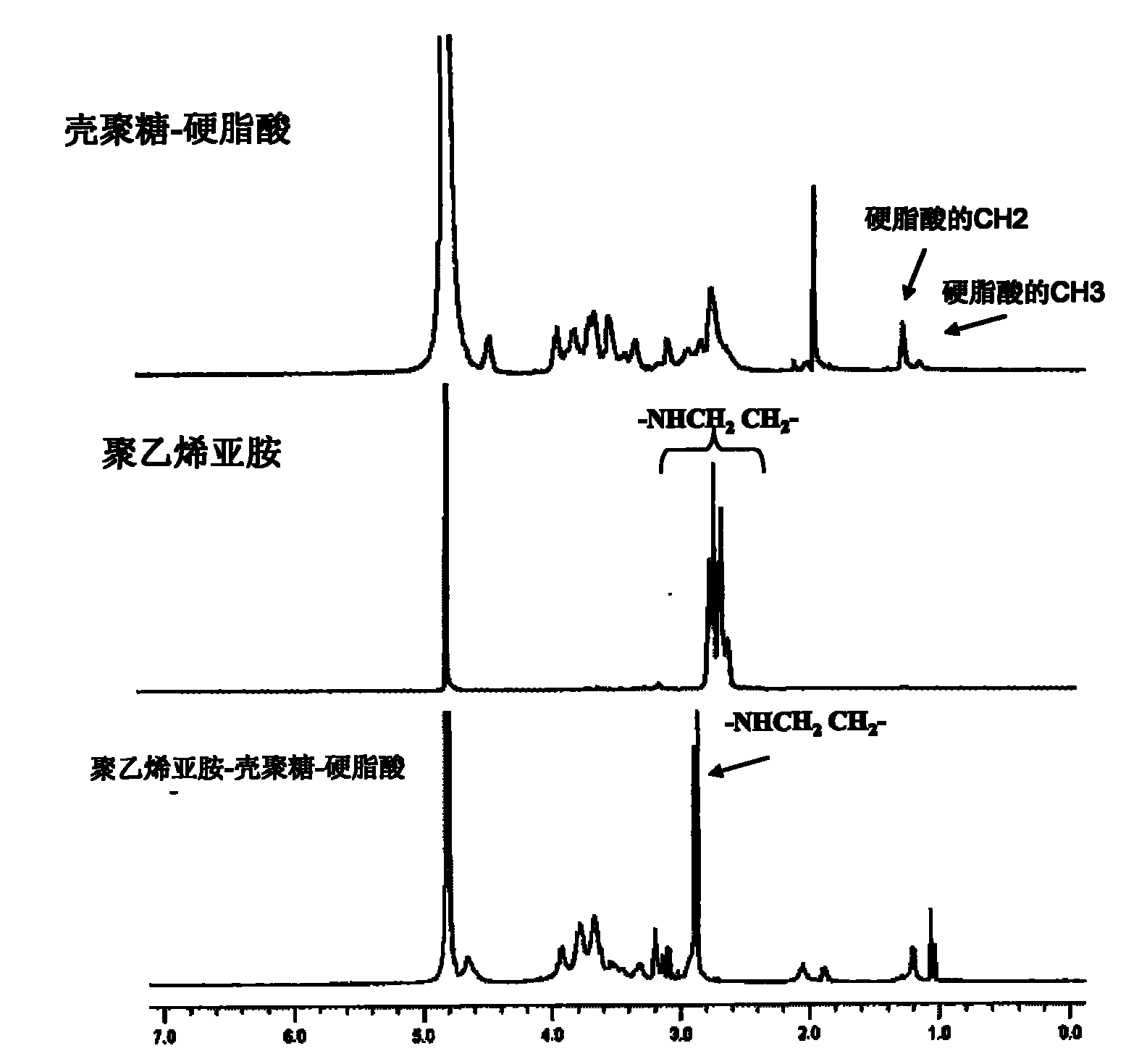
[0023] (1) Synthesis of chitosan-stearic acid graft
[0024] Accurately weigh 5.02g of chitosan whose weight-average molecular weight is 18KDa and the degree of deacetylation is 95%, add 330ml of distilled water after stirring and dissolving, add 8.27g of carbodiimide, stir and dissolve; accurately weigh 1.26g of stearic acid and dissolve in In 170ml ethanol solution, mix the above two solutions, under magnetic stirring, react at a constant temperature of 80°C for 4 hours, then cool down to room temperature, continue to stir for 6 hours until the reaction solution is clear, dialyze with distilled water for 48 hours, freeze-dry the dialysate, and use The remaining stearic acid was removed by washing with absolute ethanol to obtain a chitosan-stearic acid graft; the stearic acid grafting rate of the graft was determined to be 3.46%.
[0025] (2) Synthesis of polyethyleneimine-chitosan-stearic acid graft
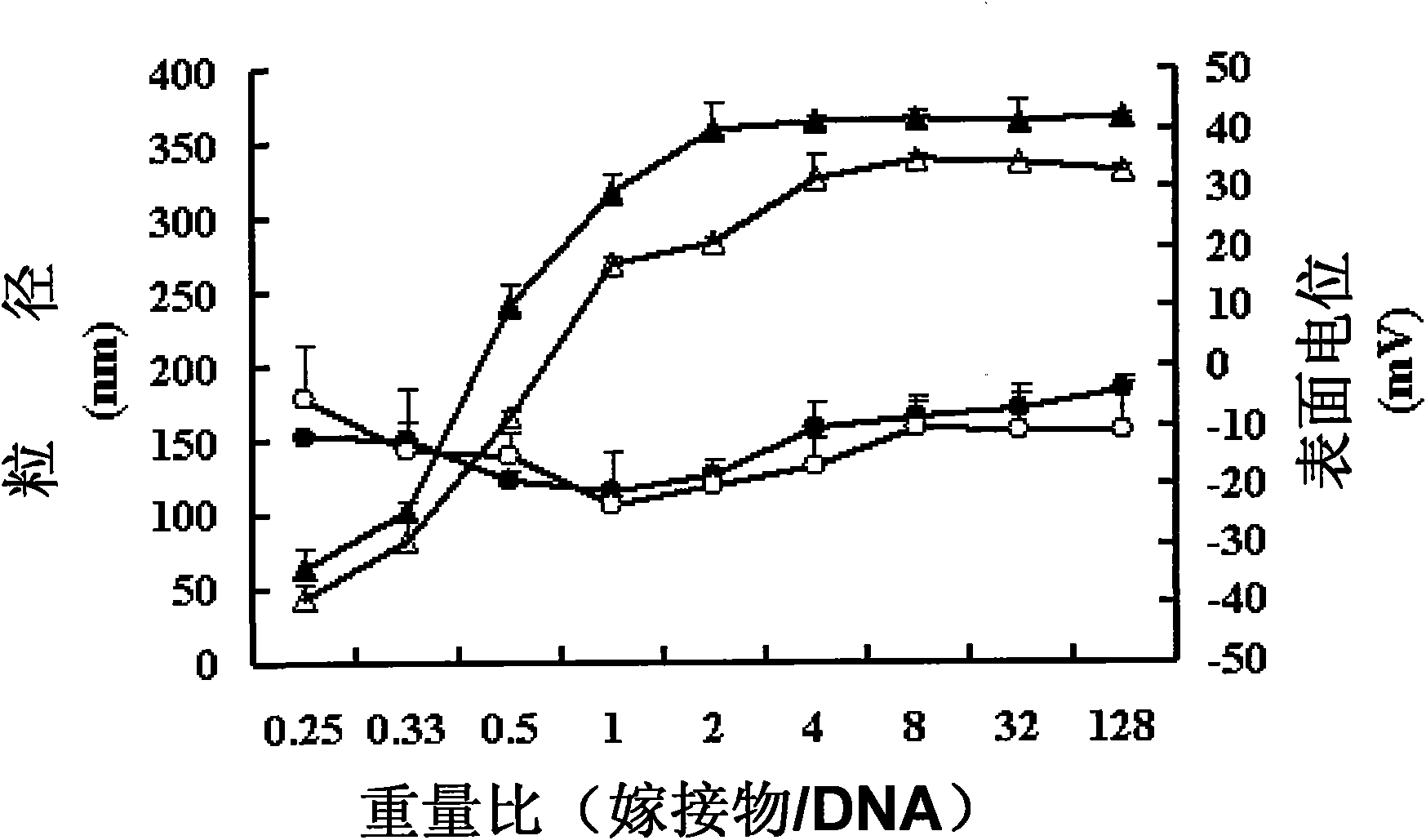
[0026]Accurately weigh 0.2 g of chitosan-stearic acid graft and 0.01 g of...
Embodiment 2
[0042] (1) Synthesis of chitosan-stearic acid graft
[0043] Accurately weigh 5.02g of chitosan whose weight-average molecular weight is 18KDa and the degree of deacetylation is 95%, add 330ml of distilled water after stirring and dissolving, add 27.56g of carbodiimide, stir and dissolve; In 170ml ethanol solution, mix the above two solutions, under magnetic stirring, react at a constant temperature of 80°C for 4 hours, then cool down to room temperature, continue to stir for 6 hours until the reaction solution is clear, dialyze with distilled water for 48 hours, freeze-dry the dialysate, and use The remaining stearic acid was removed by washing with absolute ethanol to obtain a chitosan-stearic acid graft; the stearic acid grafting rate of the graft was determined to be 20.7%.
[0044] (2) Synthesis of polyethyleneimine-chitosan-stearic acid graft
[0045] Accurately weigh 0.2 g of chitosan-stearic acid graft and 0.01 g of sodium periodate. Dissolve in 20ml of acetic acid b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com