Industrial preparation method of pantoprazole intermediate pyridine hydrochloride
A technology of pyridine hydrochloride and pantoprazole, applied in the field of medicine, can solve the problems of high cost, unfavorable industrial production, low yield and the like, and achieve the effects of easy handling, low price and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
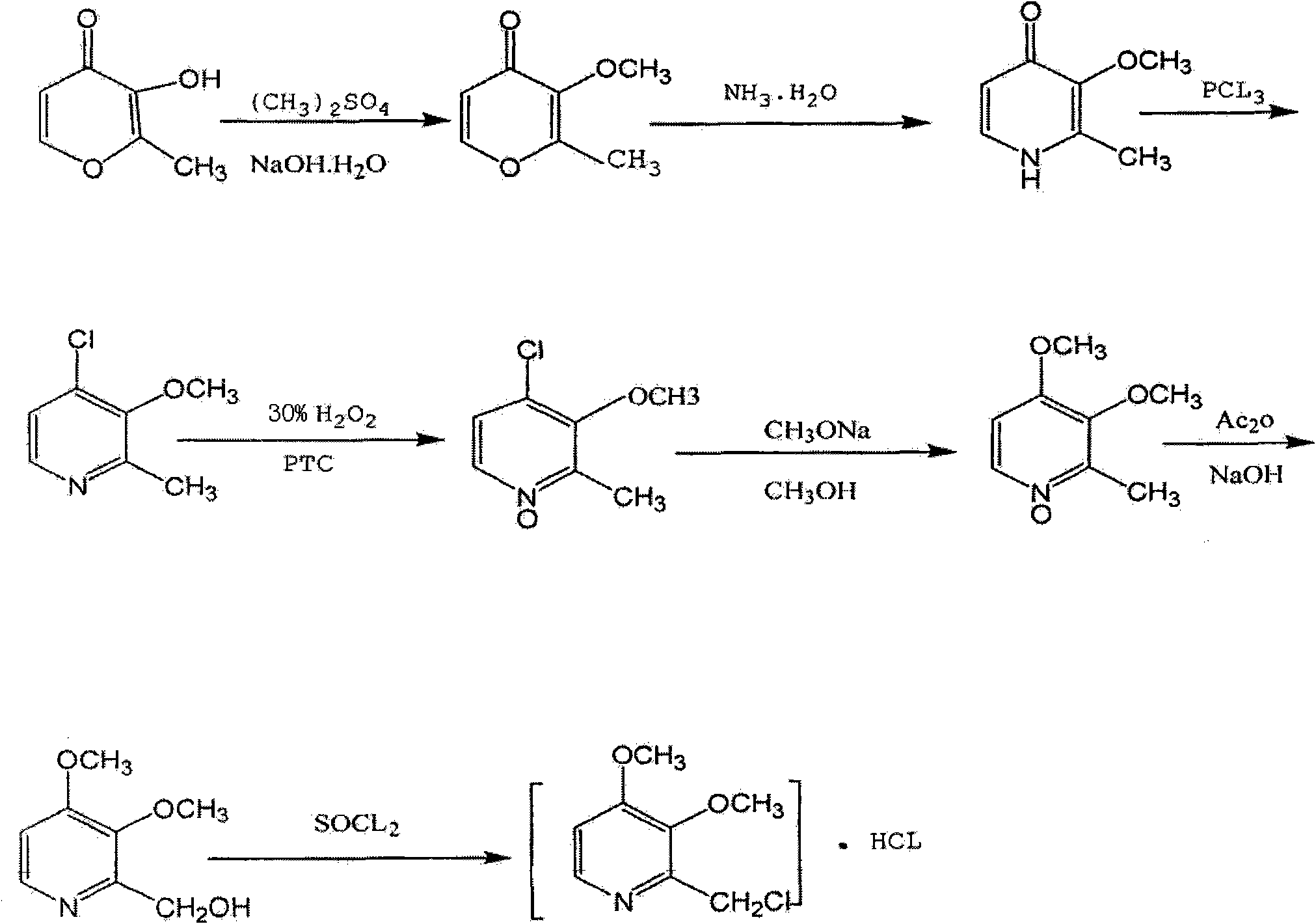
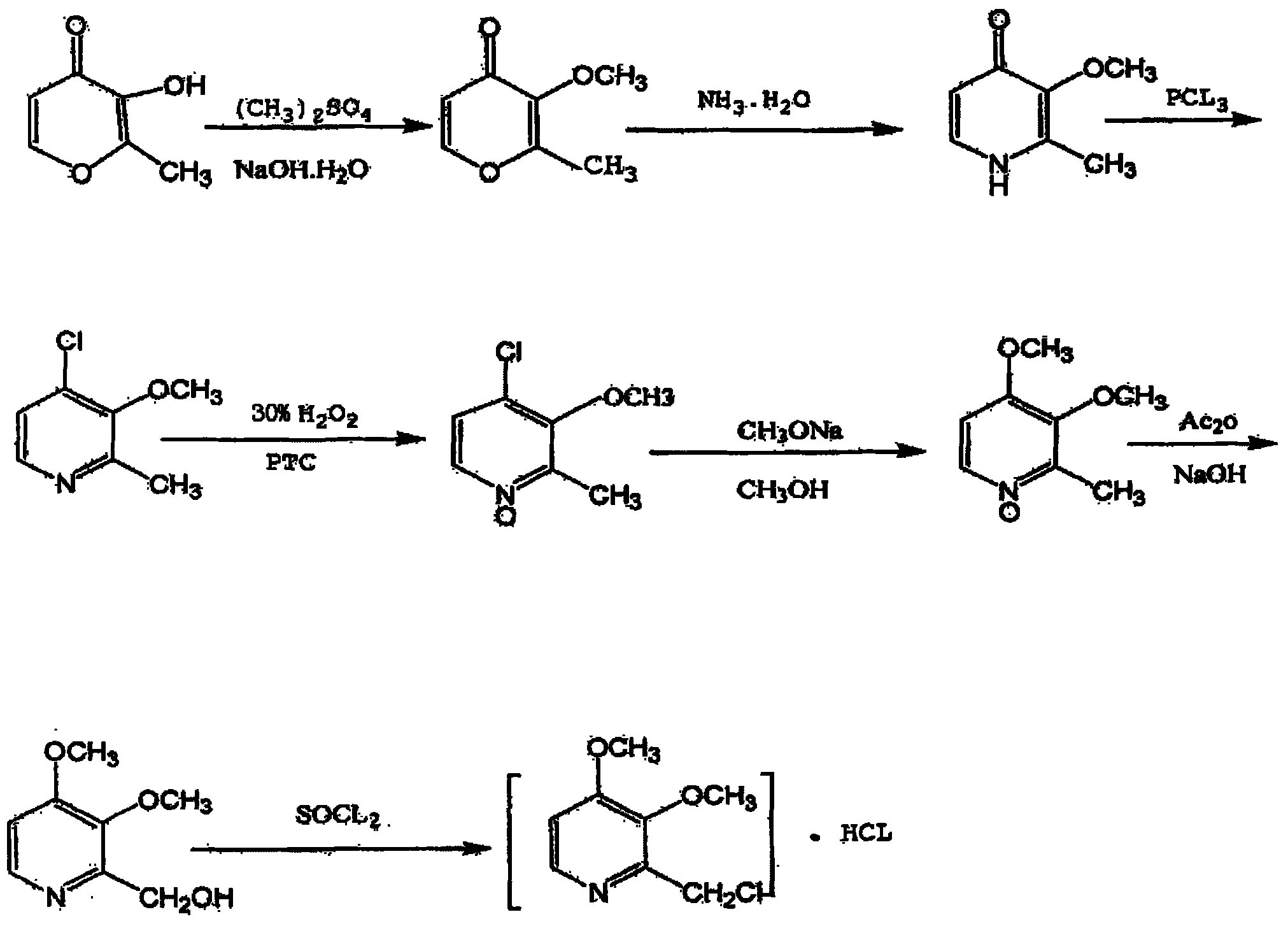
[0027] (1) Preparation of 3-methoxy-2-methyl-4H-pyran-4-one
[0028] Dissolve 140g of sodium hydroxide in 250g of water, then add 141g of maltol, stir until dissolved, raise the temperature to 84°C, slowly add 19g of dimethyl sulfate dropwise, control the dropping temperature at 84-85°C, and use 30 % sodium hydroxide solution to adjust the pH value to about 11. After the dropwise addition, the temperature was raised to 96 ° C, kept for 13 hours, and the temperature was lowered to below 30 ° C to obtain 130 g of orange-yellow liquid 3-methoxy-2-methyl-4H-pyridine Furan-4-one;
[0029] (2) Preparation of 3-methoxy-2-methyl-4(1H)-pyridone
[0030] Add 140g of 3-methoxy-2-methyl-4H-pyran-4 ketone to 1700g of ammonia water, stir at 85°C for 16 hours, then distill off the water under reduced pressure and dry to obtain 130g of brown solid 3-methoxy- 2-Methyl-4(1H)-pyridone, yield 93%;
[0031] (3) Preparation of 4-chloro-3-methoxy-2-picoline
[0032] Put 139g of 3-methoxyl-2-meth...
example 2
[0042] (1) Preparation of 3-methoxy-2-methyl-4H-pyran-4-one
[0043] Dissolve 230g of sodium hydroxide in 410g of water, then add 231g of maltol, stir until dissolved, raise the temperature to 84°C, slowly add 312g of dimethyl sulfate dropwise, control the dropping temperature at 80°C, and use 30% hydrogen Adjust the pH value of the sodium oxide solution to keep it at about 11, raise the temperature to 92°C after the dropwise addition, keep the temperature for 13 hours, and cool down to below 30°C to obtain 210g of orange-yellow liquid 3-methoxy-2-methyl-4H-pyran -4 ketone;
[0044] (2) Preparation of 3-methoxy-2-methyl-4(1H)-pyridone
[0045] Add 210g of 3-methoxy-2-methyl-4H-pyran-4one to 2550g of ammonia water, stir at 80°C for 16 hours, then distill off the water under reduced pressure and dry to obtain 198g of brown-yellow solid 3-methoxy -2-Methyl-4(1H)-pyridone, yield 93.9%;
[0046] (3) Preparation of 4-chloro-3-methoxy-2-picoline
[0047] Put 180g of 3-methoxy-2-m...
example 3
[0057] (1) Preparation of 3-methoxy-2-methyl-4H-pyran-4-one
[0058] Dissolve 160g of sodium hydroxide in 285g of water, add 159g of maltol, stir until dissolved, raise the temperature to 86°C, slowly add 218g of dimethyl sulfate dropwise, control the dropping temperature at 85°C, and use 30% hydrogen Adjust the pH value of the sodium oxide solution to keep it at about 11. After the dropwise addition, the temperature was raised to 96°C, kept for 13 hours, and the temperature was lowered to below 30°C to obtain 148g of orange-yellow liquid 3-methoxy-2-methyl-4H-pyran -4 ketone;
[0059] (2) Preparation of 3-methoxy-2-methyl-4(1H)-pyridone
[0060] Add 175g of 3-methoxy-2-methyl-4H-pyran-4one to 2100g of ammonia water, stir at 85°C for 16 hours, then distill off the water under reduced pressure and dry to obtain 164g of brown-yellow solid 3-methoxy -2-Methyl-4(1H)-pyridone, yield 93.5%;
[0061] (3) Preparation of 4-chloro-3-methoxy-2-picoline
[0062] Put 125g of 3-methoxyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com