Groove carrying-type coating decomposable drug eluting stent
A technology for eluting stents and degrading polymers, which is applied in the field of groove-carrying coating degradable vascular stents, can solve problems such as poor mechanical properties, risk of coating firmness and safety, weak adhesion performance, etc., to achieve Reduce side effects, reduce the risk of coating peeling, enhance the effect of controlled release ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
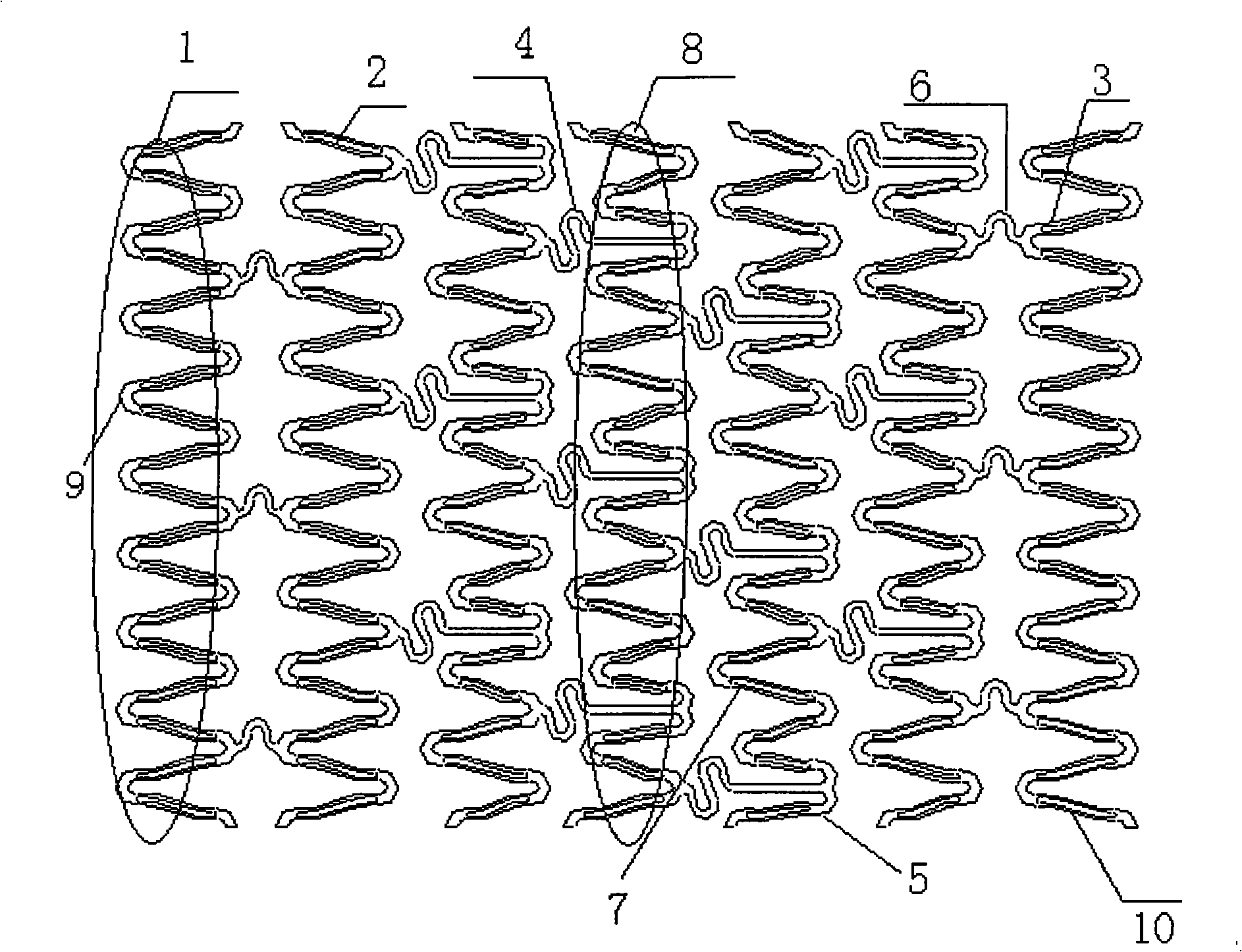
[0041] The bracket is made of cobalt-chromium alloy, the structure is like figure 1 As shown, it is composed of a plurality of main support unit rings 1, 8 and connecting rods 4, 6 connecting the unit rings. The main support unit ring is composed of a plurality of unit waves; the unit waves are composed of a circular arc section of a reinforcing ring 9 and a wave rod section The straight rod section 10 and the transition section (such as 3, 5, 7) are composed of the straight bar section (such as 3, 5, and 7). The outer surface of the unit wave bar is opened with a drug-carrying groove 2 that can be loaded with medicine; The ring width is 91μm; the transition section smoothly connects the straight rod section and the reinforcing ring; the stent thickness is 100μm.
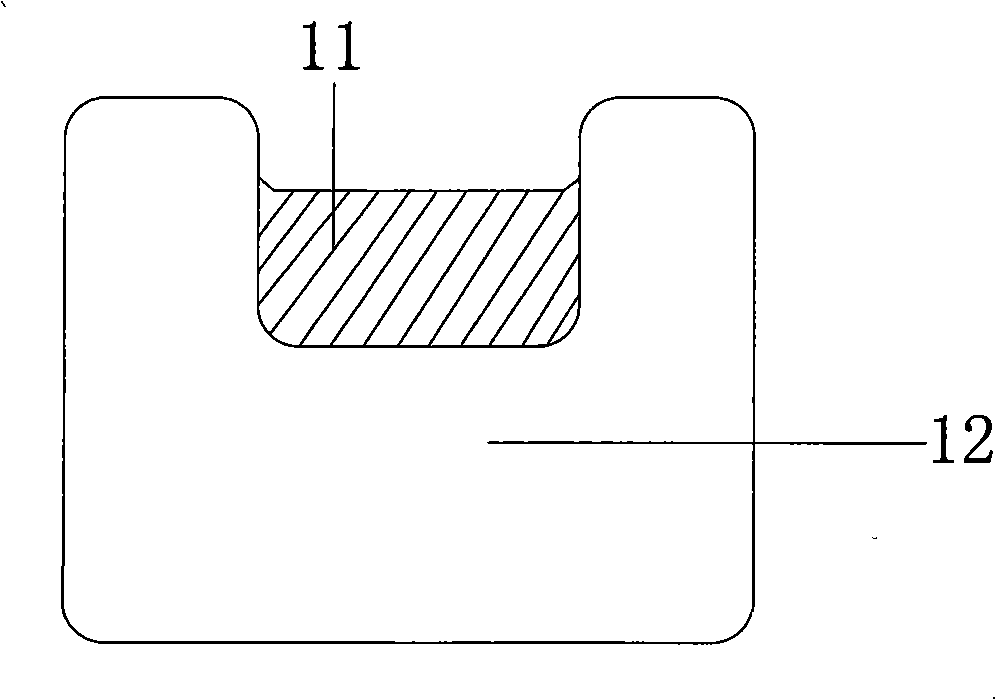
[0042] The drug-carrying groove is cut by laser cutting technology. The groove width is 60 μm and the depth is 30 μm. The cumulative groove length accounts for 60% of the total pole length of the main support unit ring....
Embodiment 2
[0046] The stent body is the same as in the first embodiment.
[0047] The drug-carrying groove is cut by laser cutting technology. The groove width is 60 μm and the depth is 30 μm. The cumulative groove length accounts for 60% of the length of the wave rod. After processing, spraying is ready for use.
[0048] Take 0.1g poly L-lactide (PLLA, weight average molecular weight range of 20,000-120,000) and 0.1g polyglycolide (PGA, weight average molecular weight range of 50,000-150,000), add 10ml tetrahydrofuran at room temperature to prepare Add 0.1g of paclitaxel to the uniform solution and mix it evenly, spray the solution accurately into the drug-carrying tank of the stent, dry the stent in a vacuum oven, and sterilize it with ethylene oxide for use. The poly-L-lactide used in Example 2 will be completely degraded within 2 years after completing the function of drug release.
Embodiment 3
[0050] The stent body is the same as in the first embodiment.
[0051] The drug-carrying groove is cut by laser cutting technology. The groove width is 55 μm and the depth is 25 μm. The cumulative groove length accounts for 65% of the length of the wave rod. After processing, spraying is ready for use.
[0052] Take 0.2g of polyglycolide-lactide (PLGA, weight average molecular weight range is 40,000-150,000), add 10ml of tetrahydrofuran at room temperature to make a uniform solution, then add 0.1g of rapamycin and mix it evenly. Spray accurately into the drug-carrying tank of the stent, dry the stent in a vacuum oven, and sterilize with ethylene oxide for use.
[0053] The polyglycolide-lactide used in Example 3 will be completely degraded within 2 years after completing the function of drug release.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com