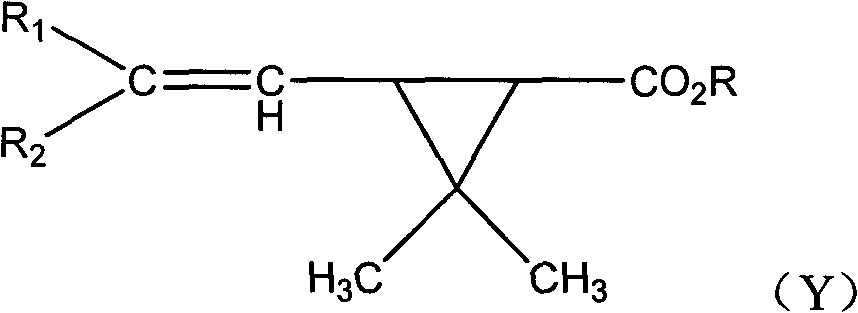
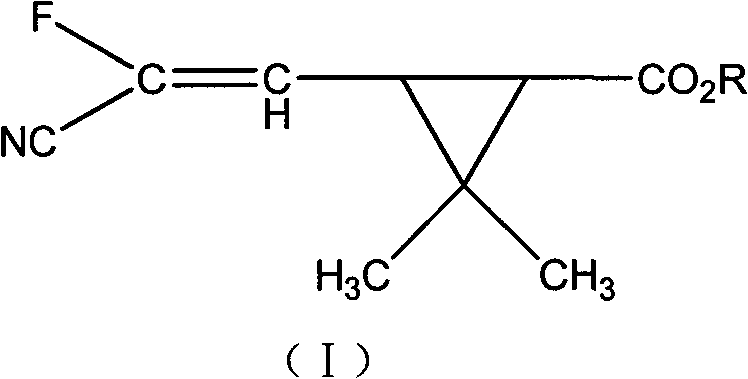
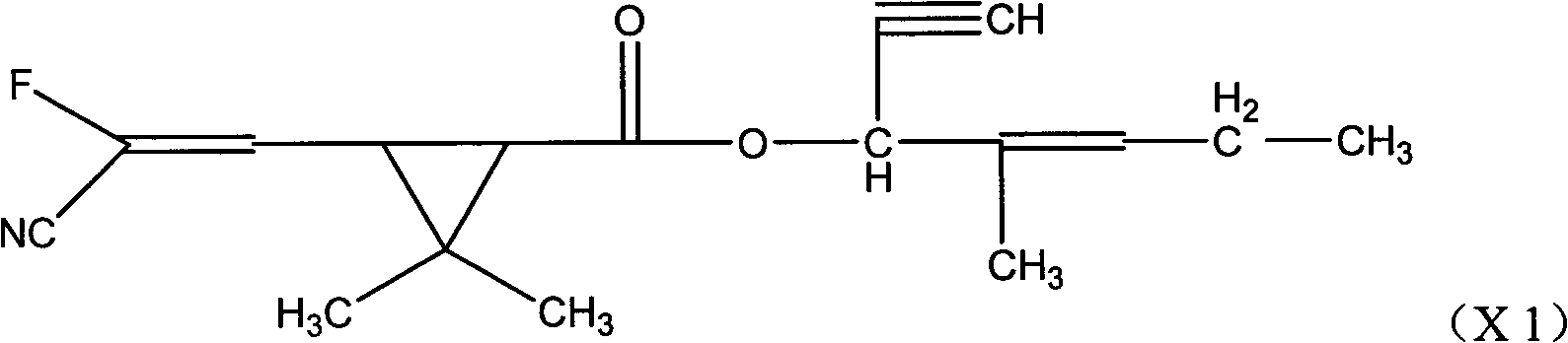
Fluorocyanogen-containing pyrethroid compound and synthesis method and application thereof
A technology for pyrethroids and compounds, which is applied in the field of cyhalothrin-containing pyrethroids and their synthesis, and achieves the effects of simple and easy processing of process routes and wastes, reduced toxicity and biological resistance, and high product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0088] Example 1.1S-cis(Z)-2,2-dimethyl-3-(2-fluoro-2-cyano-1-vinyl)-cyclopropanecarboxylic acid or 1S-cis(Z)-2, 2-Dimethyl-3-(2-fluoro-2-cyano-1-vinyl)-cyclopropaneyl chloride
[0089] (1) Preparation of 1S-cis(Z)-2,2-dimethyl-3-(2-fluoro-2-cyano-1-vinyl)cyclopropanecarboxylic acid
[0090] 0.5 g of p-toluenesulfonic acid (PTSA) was added to dissolve 6.00 g of 1R-cis(Z)-2,2-dimethyl-3-(2-fluoro-2-cyano-1-vinyl)cyclopropane Carboxy-1,1-dimethylethyl ester and 50 ml of toluene. React until heated to reflux at 120°C. Then cool down to 20 degrees, dilute with isopropyl ether, wash with water, dry, filter and concentrate to obtain 4.60g 1S-cis(Z)-2,2-dimethyl-3-(2-fluoro-2-cyano- 1-vinyl)cyclopropanecarboxylic acid.
[0091] (2) Preparation of 1S-cis(Z)-2,2-dimethyl-3-(2-fluoro-2-cyano-1-vinyl)-cyclopropaneyl chloride
[0092] 1ml DMF (N,N-dimethylformamide) and 4.5ml (COCl) 2 (Thionyl chloride) was added to 0°C to dissolve 4.60g 1S-cis(Z)-2,2-dimethyl-3-(2-fluoro-2-cyano-1-...
Embodiment 21
[0093] Example 2.1 R-trans(E)-2,2-dimethyl-3-(2-fluoro-2-cyano-1-vinyl)-cyclopropanecarboxylic acid or 1R-trans(E)-2, 2-Dimethyl-3-(2-fluoro-2-cyano-1-vinyl)-cyclopropaneyl chloride:
[0094] (1) Preparation of 1R-trans(E)-2,2-dimethyl-3-(2-fluoro-2-cyano-1-vinyl)-cyclopropanecarboxylic acid:
[0095] 1.2 g of p-toluenesulfonic acid (PTSA) was added dissolved in 14.15 g of 1R-trans(E)-2,2-dimethyl-3-(2-fluoro-2-cyano-1-vinyl)cyclopropane Carboxy-1,1- Dimethyl ethyl ester and 140ml of toluene. React until heated to reflux at 120°C. Then cool down to 20°C, dilute with 250ml of isopropyl ether, wash with water, dry, filter and concentrate to obtain 11.32g of 1R-trans(E)-2,2-dimethyl-3-(2-fluoro-2-cyano- 1-vinyl)cyclopropanecarboxylic acid.
[0096] (2) Preparation of 1R-trans(E)-2,2-dimethyl-3-(2-fluoro-2-cyano-1-vinyl)-cyclopropaneyl chloride:
[0097] 3ml DMF and 10ml of (COCl) 2 10 g of 1R-trans(E)2,2-dimethyl-3-(2-fluoro-2-cyano-1-vinyl)cyclopropanecarboxylic acid and 10...
Embodiment 3
[0099] The synthesis of embodiment 3 compound (X1)
[0100] In a 500ml four-necked reaction flask, put 31.0g of 1-ethynyl-2-methylpent-2-enol, 25.0g of pyridine, dissolve in 200ml of toluene, stir well, add 3- (2-Fluoro-2-cyano-1-vinyl)-2,2-dimethylcyclopropanecarboxylic acid chloride 56.0 g, after the dropwise addition, the temperature was raised to 25° C. for 2 hours to react. Wash twice with 150ml 10% hydrochloric acid, then wash with 150ml 10% NaHCO 3 Wash twice, separate the toluene layer and heat at 0.08Mpa to remove the solvent toluene to obtain -1-ethynyl-2-methylpent-2-enyl-3-(2-chloro-3,3,3-tri Fluoroallyl)-2,2-dimethylcyclopropane carboxylate, the weight is 65.7g, the content is 92.3%, and the yield is 83.9%.
[0101]
[0102] The IR spectrum shows υ-c=o 1750cm -1 The strong absorption peak at and υ-c-o-c-1180cm -1 and 1160cm -1 The two strong peaks of the product indicate the presence of ester groups; υ-c=o at 1720cm -1 The strong and broad peaks on the le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com