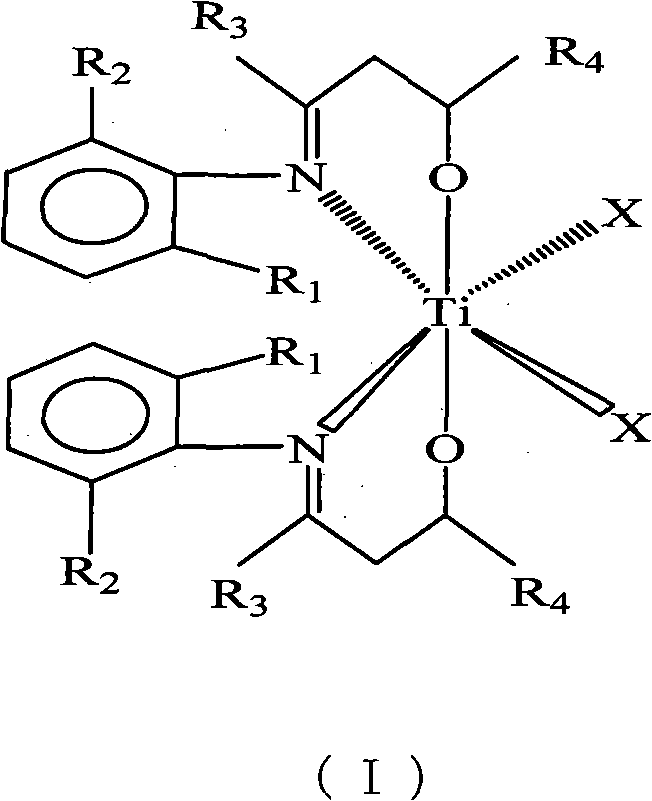
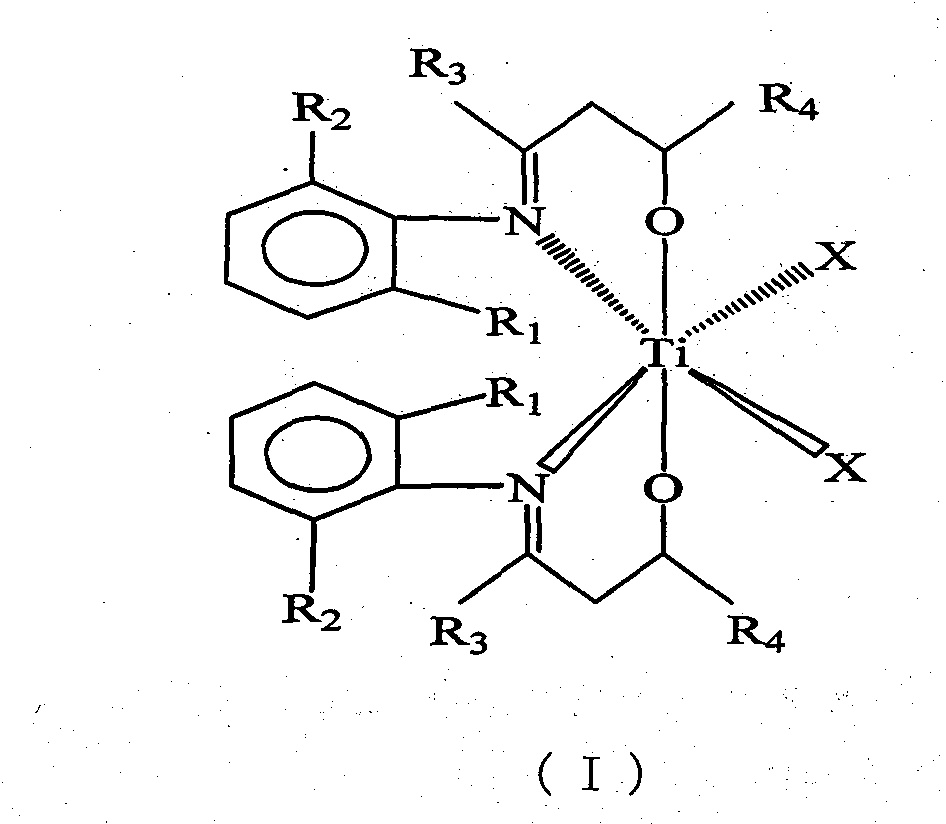
Beta-hydroxy imine titanium metal catalyst and method for polymerizing ethylene
A technology of metal catalyst, titanium hydrazine, applied in the direction of titanium organic compound, imino compound preparation, organic chemistry, etc., can solve the problem of low activity of ethylene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] C 6 h 5 N=C(CH 3 )CH 2 Synthesis of CH(Ph)OH Ligand
[0018] Under the protection of an inert gas, 22.32ml (0.0210mol) of lithium diisopropylamide and 25.00ml of tetrahydrofuran were added to a 250ml three-necked flask, and 2.79g (0.0210mol) of C 6 h 5 N=C(CH 3 )CH 3 25.00ml tetrahydrofuran solution, the temperature rises naturally, and the reaction is stirred for 0.5 hours. At -5°C, a solution of 1.43ml (0.0140mol) benzaldehyde in 25.00ml tetrahydrofuran was added and reacted for 10 hours. Pour the reaction solution into ice water, use a separatory funnel to separate the liquid, remove part of the solvent from the organic phase, put it in the refrigerator for crystallization, and use an organic solvent composed of n-hexane and ethyl acetate at a volume ratio of 1:1 After recrystallization, 1.34 g of solid was obtained with a yield of 40%.
[0019] [C 6 h 5 N=C(CH 3 )CH 2 CH(Ph)O] 2 TiCl 2 Synthesis
[0020] Under inert gas protection, in the Schlenk bo...
Embodiment 2
[0022] (2-CH 3 -C 6 h 4 )N=C(C 4 h 9 )CH 2 Synthesis of CH(Ph)OH
[0023] Under the protection of inert gas, 22.32ml (0.0210mol) lithium diisopropylamide and 25.00ml toluene were added to a 250ml three-necked flask, and 2.65g (0.0140mol) (2-CH 3 -C 6 h 4 )N=C(C 4 h 9 )CH 3 25.00ml of toluene solution, the temperature rises naturally, and the reaction is stirred for 1 hour. At 0°C, a solution of 1.43ml (0.0140mol) benzaldehyde in 25.00ml toluene was added and reacted for 20 hours. Pour the reaction solution into ice water, separate the liquid with a separatory funnel, remove part of the solvent from the organic phase, put it in the refrigerator for crystallization, and recrystallize the precipitated crystals with an organic solvent composed of n-hexane and toluene at a volume ratio of 5:1 , 1.74 g of solid was obtained, yield 42%.
[0024] [(2-CH 3 -C 6 h 4 )N=C(C 4 h 9 )CH 2 CH(Ph)O] 2 TiCl 2 Synthesis
[0025]Under inert gas protection, 0.68g (2.32mmol)...
Embodiment 3
[0027] (2-CH 3 -6-C 2 h 5 -C 6 h 3 )N=C(Ph)CH 2 C(2-CH 3 Synthesis of O-Ph)OH
[0028] Under the protection of inert gas, 22.32ml (0.0210mol) lithium diisopropylamide and 25.00ml ether were added to a 250ml three-necked flask, and 2.49g (0.0105mol) (2-CH 3 -6-C 2 h 5 -C 6 h 3 )N=C(Ph)CH 3 25.00ml tetrahydrofuran solution, the temperature rises naturally, and the reaction is stirred for 2 hours. At 5°C, add 0.64ml (0.0053mol) p-methoxybenzaldehyde in 25.00ml ether solution, and react for 24 hours. Pour the reaction solution into ice water, separate the liquid with a separatory funnel, remove part of the solvent from the organic phase, put it in the refrigerator for crystallization, and recrystallize the precipitated crystals with an organic solvent composed of n-hexane and tetrahydrofuran at a volume ratio of 8:1 , 0.85 g of solid was obtained with a yield of 43%.
[0029] [(2-CH 3 -6-C 2 h 5 -C 6 h 3 )N=C(Ph)CH 2 C(2-CH 3 O-Ph)O] 2 TiBr 2 Synthesis
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com