Production process for isolating fluorine and chlorine by acid extraction during electrolytic zinc production
A production process, electrolytic zinc technology, applied in the direction of photographic process, photographic auxiliary process, process efficiency improvement, etc., can solve the problems of increased fluorine and chlorine concentration, high fluorine and chlorine raw materials cannot be effectively treated, etc., to reduce processing costs, Promoting the effective utilization of secondary resources and improving the metal recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
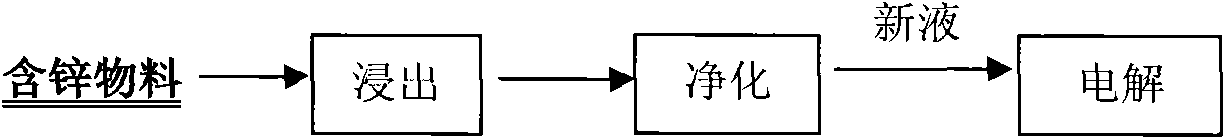
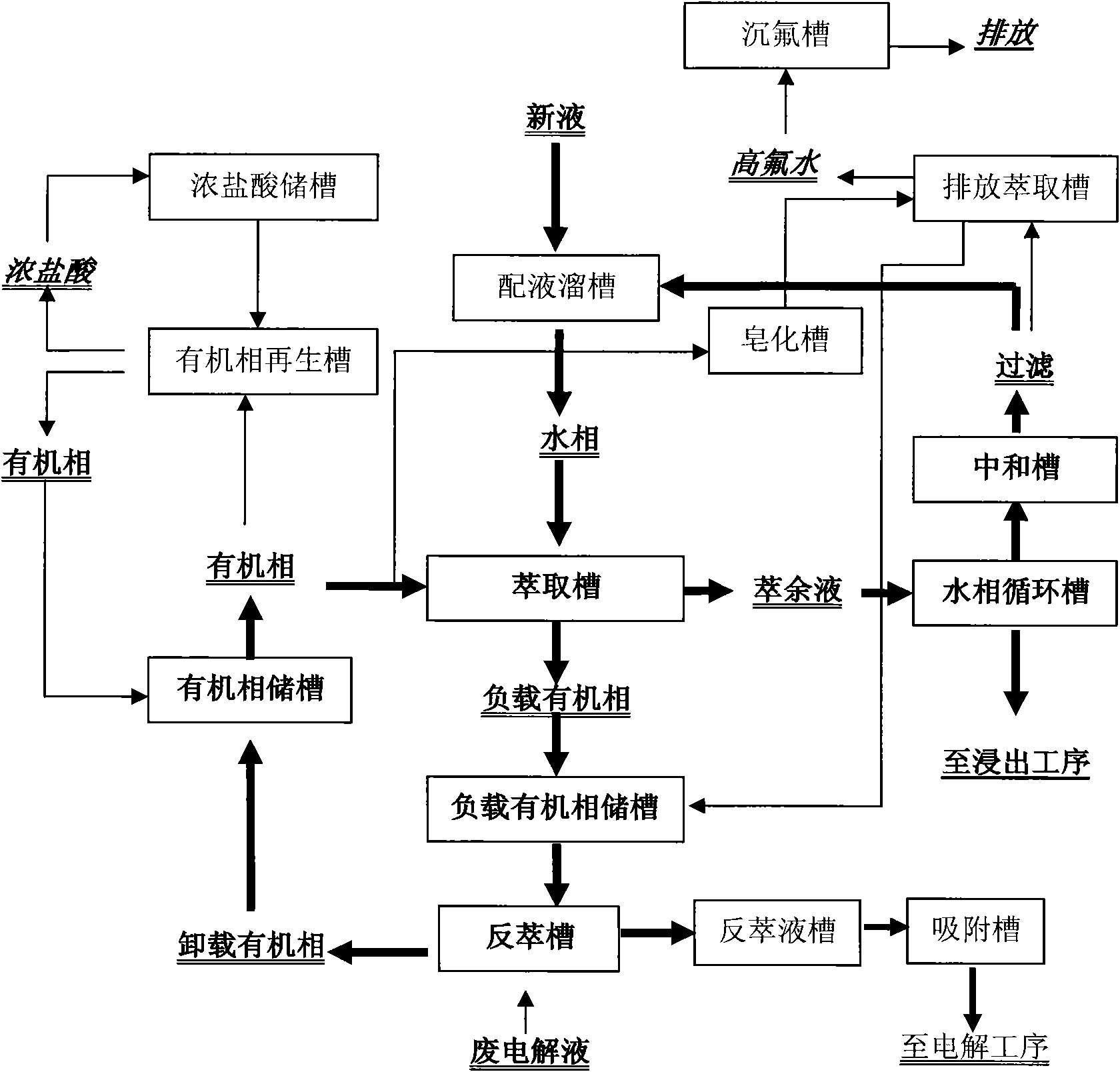
[0033] For the leaching-purification-electrolysis process that has been adopted in the hydrometallurgy (see figure 1) enterprises only need to establish a corresponding extraction system near the original system according to the design plan, introduce the original new liquid pipeline into the liquid distribution tank of the water phase circulation system of the extraction system, and introduce the circulating electrolyte into the reverse extraction system according to the required flow rate of the reverse extraction The extraction system can be realized without any changes to the original system. For the new system, the system design can be carried out according to the traditional electrolytic zinc production process, and it can be realized by adding an extraction system between the purification process and the electrolysis process (see figure 2 ).
[0034] The specific steps are as follows: (see figure 2 , image 3 ).
[0035] 1. Using zinc calcined sand or zinc oxide o...
Embodiment 2
[0051] Repeat the same steps described in Example 1, but the process parameters are adjusted as follows: two (2-ethylhexyl) phosphoric acid (trade name P204) is carried out by volume ratio 10-19% and kerosene by volume ratio 81-89% Mix to form the organic phase for extraction; control the concentration of zinc ions in the water phase to 10-20g / l, adjust the pH value to 5; extraction conditions: the ratio of the organic phase to the water phase is 1 / 1, the mixing time is 5 minutes, and the temperature is controlled at 10°C-15°C, the pH value of the extraction raffinate is 5; stripping conditions: use the waste electrolyte with a sulfuric acid concentration of 160-180g / l and a zinc ion concentration of 45-55g / l for stripping, the organic phase and the waste electrolyte The stripping ratio is 4 / 1, and the stripping temperature is 10°C-20°C; saponification is carried out with 25% sodium hydroxide solution; stripping regeneration is carried out with 19-25% concentrated hydrochloric ...
Embodiment 3
[0053] Repeat the same steps described in Example 1, but the process parameters are adjusted as follows: two (2-ethylhexyl) phosphoric acid (trade name P204) is carried out by volume ratio 19-25% and kerosene by volume ratio 75-81% Mix to form the organic phase for extraction; control the concentration of zinc ions in the water phase to 20-30g / l, and adjust the pH value to 6; extraction conditions: the organic phase is 1 / 2 of the water phase, the mixing time is 7 minutes, and the temperature is controlled at 15°C-25°C, the pH value of the extraction raffinate is 5; stripping conditions: stripping with waste electrolyte with sulfuric acid concentration of 180-200g / l and zinc ion concentration of 55-70g / l, organic phase and waste electrolyte The stripping ratio is 5 / 1-7 / 1, and the stripping temperature is 20°C-30°C; saponification with 35% sodium hydroxide solution; stripping regeneration with 25-32% concentrated hydrochloric acid .
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com