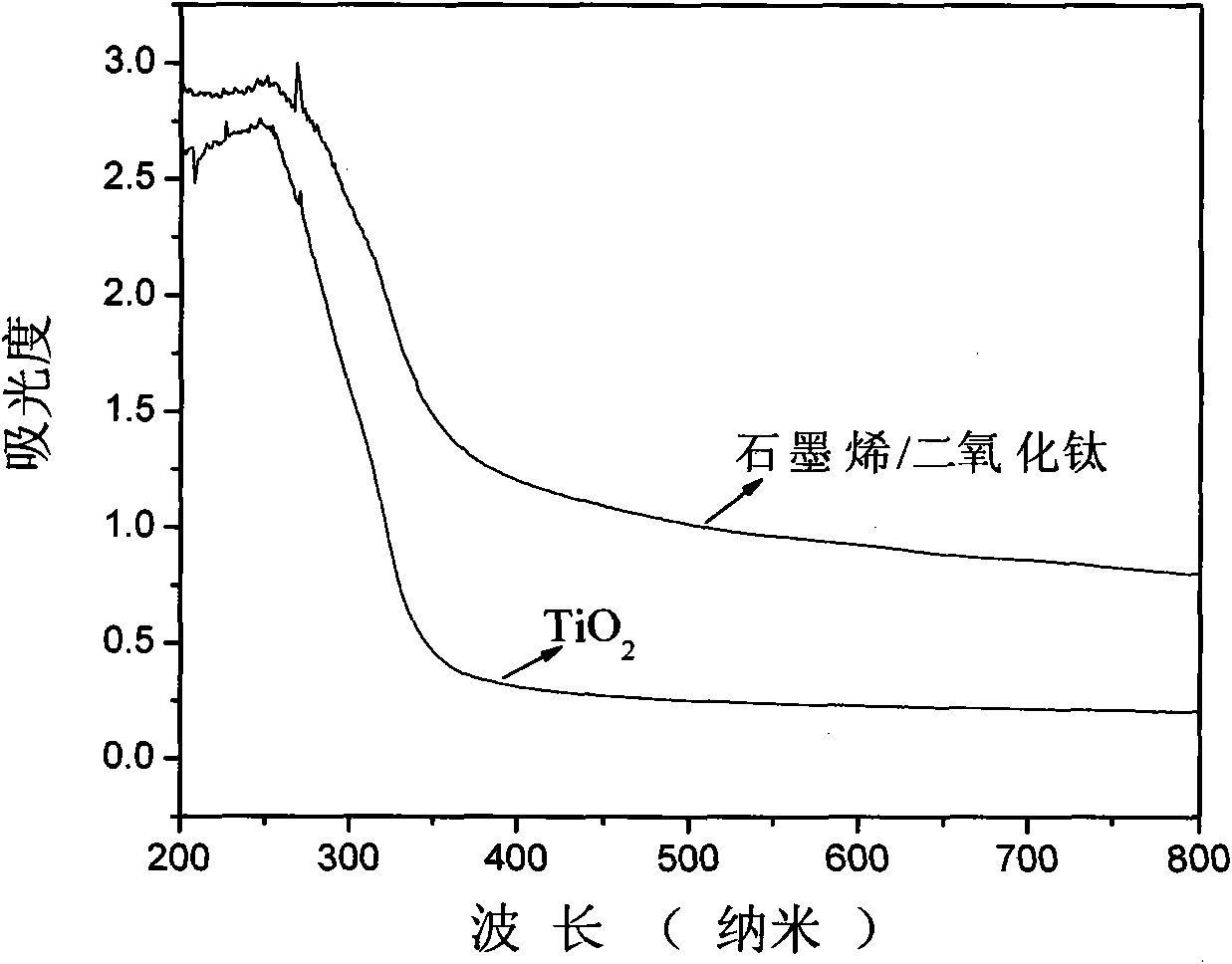
Preparation method of graphene/titanium dioxide composite photocatalyst
A technology of titanium dioxide and composite light, which is applied in the field of photocatalysis to achieve excellent photocatalytic activity, avoid agglomeration, and prevent heavy accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 30 mg of graphite oxide to 30 mL of isopropanol, and sonicate for 1 hour to obtain a graphene oxide dispersion. Then 5 mL of n-butyl titanate was added and stirred for 30 minutes, followed by the addition of 1 mL of deionized water and continued stirring for 30 minutes to obtain a beige gel. The gel was transferred to a hydrothermal reactor and reacted at 180°C for 8 hours. The hydrothermal product was washed several times by centrifugation with ethanol and deionized water respectively, placed in a vacuum oven, and dried at 60° C. for 12 hours to obtain a graphene / titanium dioxide composite photocatalyst.
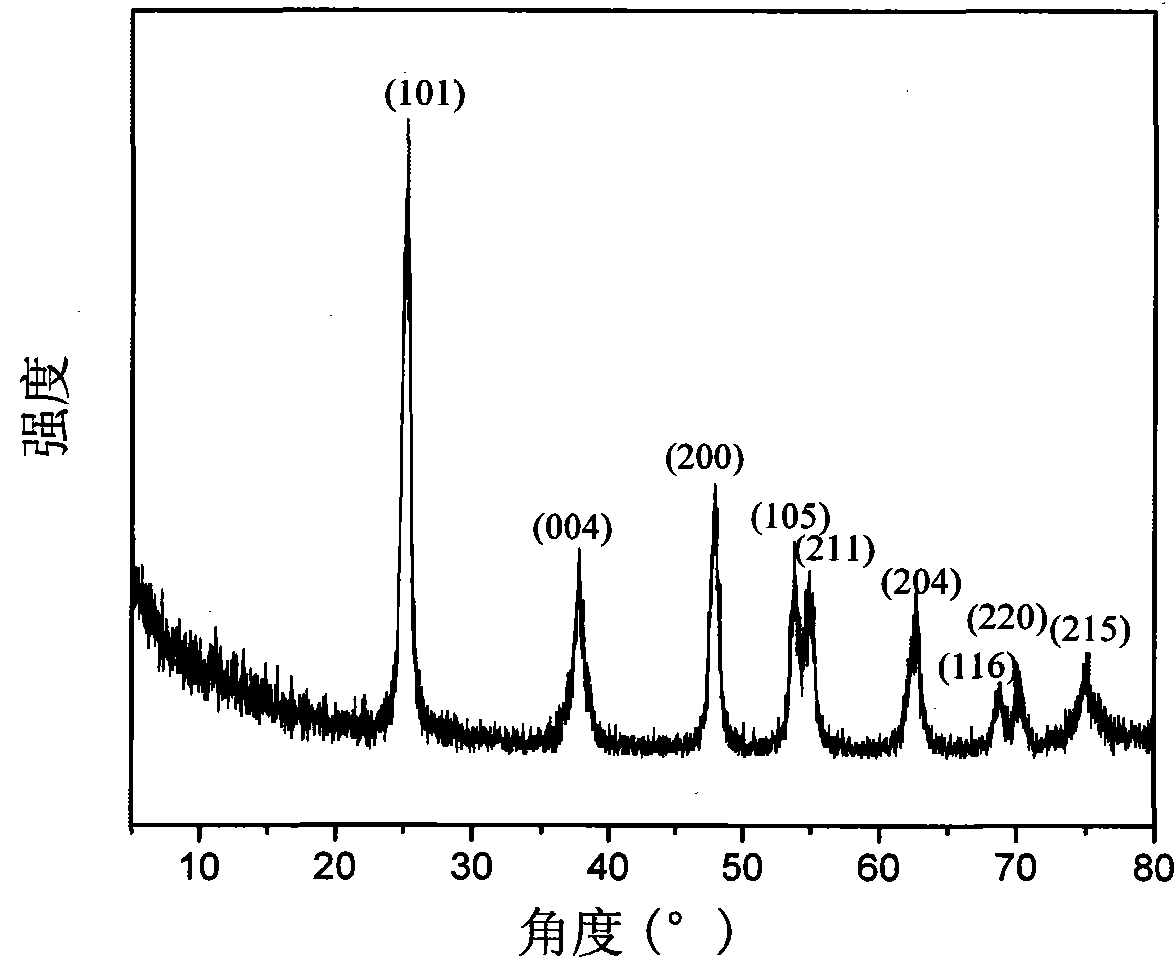
[0020] figure 1 The XRD figure of the graphene / titanium dioxide composite material that is made in this embodiment. All the diffraction peaks in the figure correspond to TiO 2 In the anatase phase, the characteristic diffraction peak of graphite oxide at 2θ=10.7° disappeared, indicating that the hydrothermal reaction effectively reduced graphite oxide to graph...
Embodiment 2
[0022] Add 10 mg of graphite oxide to 30 mL of isopropanol, and sonicate for 40 minutes to obtain a graphene oxide dispersion. Then 5 mL of n-butyl titanate was added and stirred for 30 minutes, followed by the addition of 1 mL of deionized water and continued stirring for 20 minutes to obtain a beige gel. The gel was transferred to a hydrothermal reactor and reacted at 140°C for 12 hours. The hydrothermal product was washed several times by centrifugation with ethanol and deionized water respectively, placed in a vacuum oven, and dried at 50° C. for 18 hours to obtain a graphene / titanium dioxide composite photocatalyst.
Embodiment 3
[0024] Add 50 mg of graphite oxide to 30 mL of ethanol, and ultrasonicate for 1 hour to obtain a graphene oxide dispersion. Then 5 mL of n-butyl titanate was added and stirred for 30 minutes, followed by the addition of 1 mL of deionized water and continued stirring for 30 minutes to obtain a beige gel. The gel was transferred to a hydrothermal reactor and reacted at 200°C for 6 hours. The hydrothermal product was washed several times by centrifugation with ethanol and deionized water respectively, placed in a vacuum oven, and dried at 70° C. for 10 hours to obtain a graphene / titanium dioxide composite photocatalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com