Derivate for labeling bile acid and reference compound, preparation method and application thereof
A technology of bile acid derivatives and compounds, applied in the field of labeling drug derivatives and their reference compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
[0166] The synthesis of embodiment 5 formula V compound
[0167]
[0168] (1) Synthesis of N-ethylamino[bis(2-methylenepyridine)]cholic acid amide tridentate tricarbonyl rhenium chelate
[0169] Among them, A represents -(CH 2 CH 2 ) x -, x=1, R 1 = H, R 2 =α-OH, R 3 =α-OH, the specific synthesis steps are as follows: 100mg (0.158mmol) III was dissolved in 5mL of absolute ethanol, then added (Et 4 N) 2 [Re(CO) 3 Br 3 ]121mg (0.158mmol), reacted at room temperature (25°C) for 1h, evaporated under reduced pressure to remove the solvent, and separated by plate chromatography to obtain 98mg, yield 70%, m.p.125.7~127.4°C.
[0170] The identification data are as follows:
[0171] IR(KBr): v(O-H)3416, v(C=O)1642, v(C=N)1405cm -1 ;
[0172] 1 H NMR (500MHz, CD 3 OD, δ): 0.70(s, 3H, 18-CH3), 0.91(s, 3H, 19-CH 3 ), 0.92~0.98(m, 1H), 1.05(d, 3H, J=6.3Hz, 21-CH3), 1.10~1.17(m, 1H), 1.22~1.30(m, 2H), 1.33~1.46(m , 5H), 1.51~1.68(m, 6H), 1.71~2.08(m, 8H), 2.20~2.38(m, 4H)...
Embodiment 699m
[0226] Example 6 99m Radiochemical Synthesis of Formula III Compounds by Tc
[0227] 1. fac-[ 99m Tc(CO) 3 (H 2 O) 3 ] + radiochemical synthesis of
[0228]
[0229] (a) NaBH 4 , Na 2 CO 3 , potassium sodium tartrate, 30min;
[0230] fac-[ 99m Tc(CO) 3 (H 2 O) 3 ] + The synthesis of is mainly according to the method of the literature (Journal of Pharmaceutical and Biomedical Analysis, 2007, 43: 1576-1579.), with slight modifications.
[0231] a.15.0mg KNaC 4 h 4 o 6 4H 2 O, 4.0 mg anhydrous Na 2 CO 3 6.0 mg NaBH 4 , mixed in a 10 mL vial.
[0232] b. Charge CO into it for 30 minutes.
[0233] c. Add 1-2ml of freshly rinsed 99m TCO 4 - Solution (10mCi), 70-80 ℃ water bath 30min, ice-water bath cooling, namely fac-[ 99m Tc(CO) 3 (H 2 O) 3 ] + .
[0234] 2. Using fac-[ 99m Tc(CO) 3 (H 2 O) 3 ] + Steps for radiolabeling N-ethylamino[bis(2-methylenepyridine)]cholic acid amide ligand:
[0235] take fac-[ 99m Tc(CO) 3 (H 2 O) 3 ] + Solut...
Embodiment 799m
[0236] Example 7 99m Radiochemical Synthesis of Formula III Compounds by Tc
[0237] 1. fac-[ 99m Tc(CO) 3 (H 2 O) 3 ] + radiochemical synthesis of
[0238] The method and conditions are all the same as (1) in Example 6.
[0239] 2. Using fac-[ 99m Tc(CO) 3 (H 2 O) 3 ] + Steps for radiolabeling N-ethylamino[bis(2-methylenepyridine)]cholic acid amide ligand:
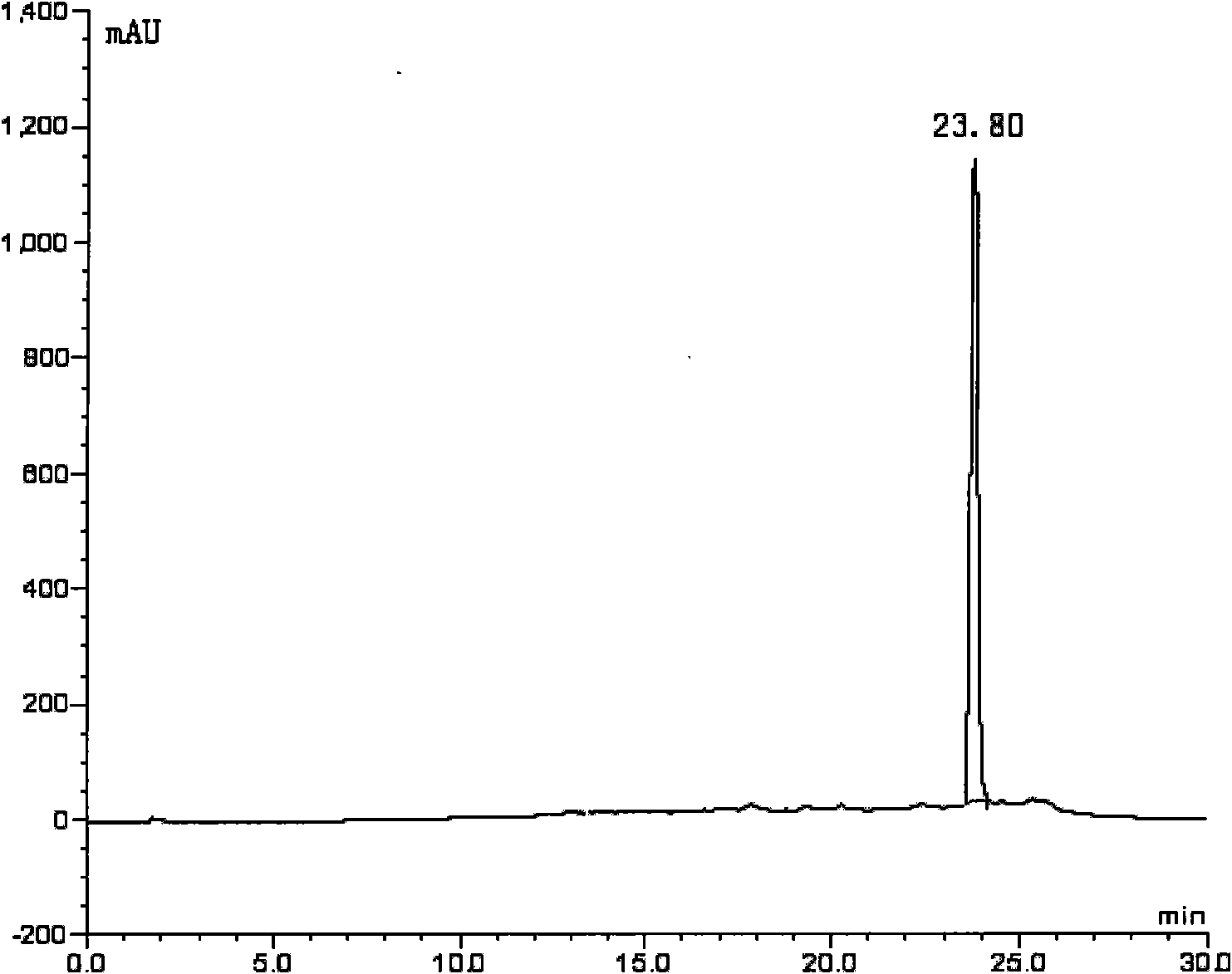
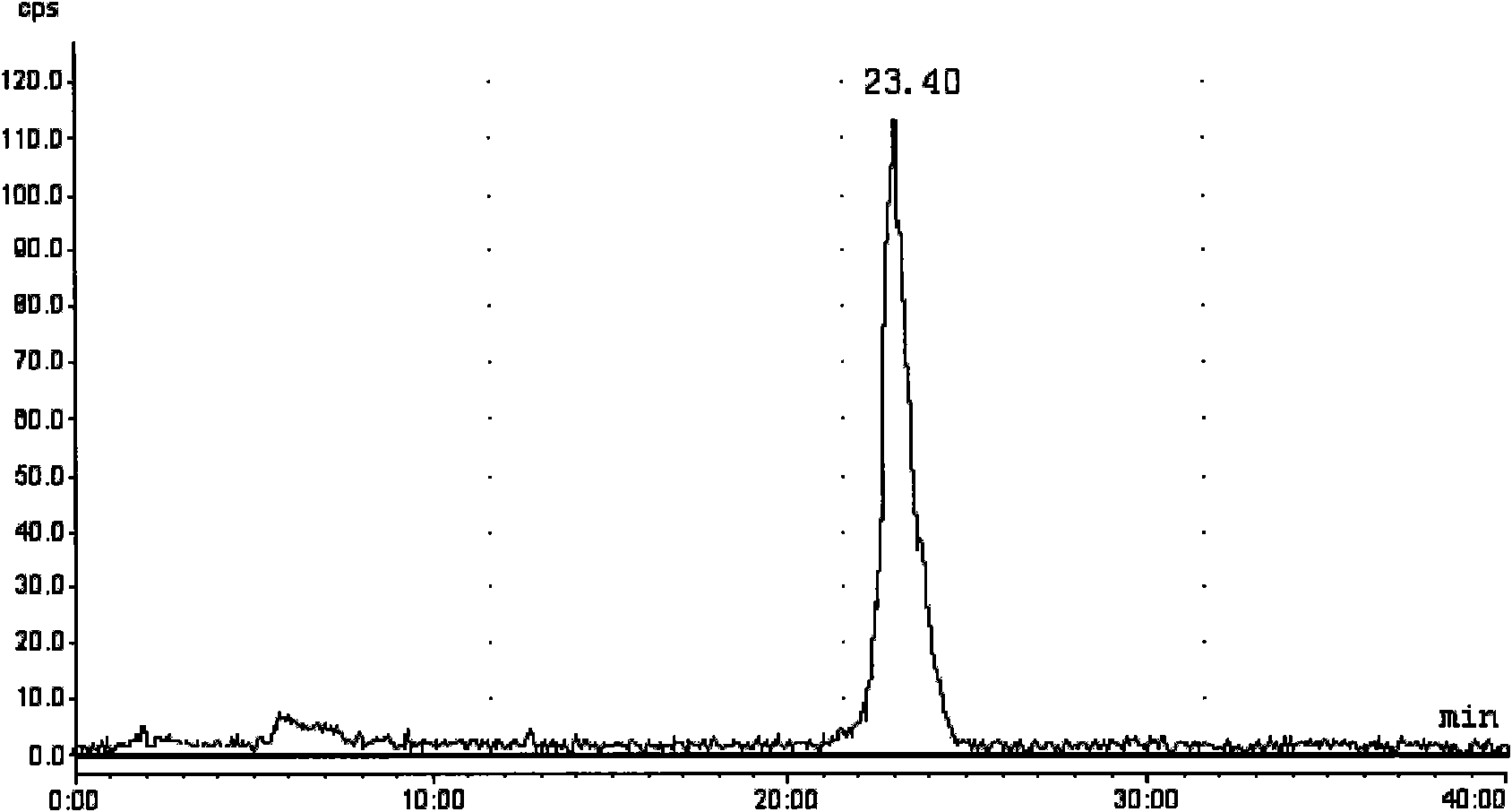
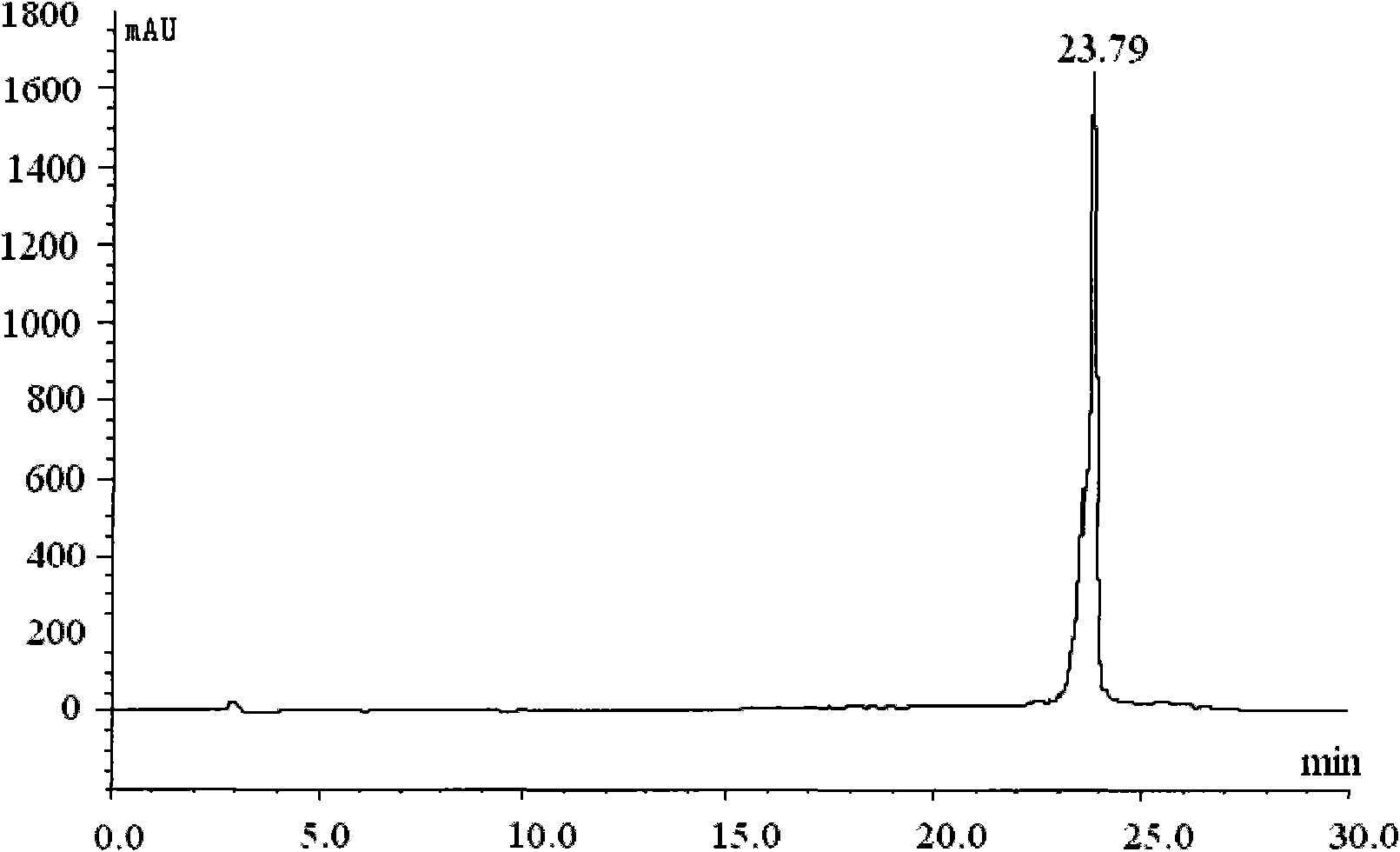
[0240] take fac-[ 99m Tc(CO) 3 (H 2O) 3 ] + Solution 0.4ml (1.2mci), add 10 -3 mol / L Compound III N-ethylamino[bis(2-methylenepyridine)]cholic acid amide in dimethyl sulfoxide solution 1.2ml, sealed with a parafilm, placed on an incubator, temperature controlled at 65°C, Rotating speed 1000 rev / mins, time 30min, then adopt high performance liquid chromatography (HPLC) [HPLC system: Merck-Hitachi L-7000 system (connected with UV detector and EG&G Berthold LB 508 radioactivity detector), chromatographic column is C- 18ec reverse-phase column, Ф3mm*250mm, 5μm; HPLC elution gradient (solution A: anhydrous m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com