C crystal form solid matter of bergenin and preparation method and application thereof
A technology of petracenin and solid substances, which is applied in the field of medicine and can solve the problems of undiscovered petracenin crystal form patents or literature reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0065] Preparation example The preparation of B-type sample of petogenin crystal
[0066] Take 5g of petracenin sample, completely dissolve the petracenin sample with tetrahydrofuran solvent at room temperature of 20°C, heat it to a temperature of 40°C, evaporate the solvent quickly under vacuum conditions, and prepare the obtained petracenin 4.40 g of crystalline solid substance. HPLC purity 98.7%, recovery 88%.
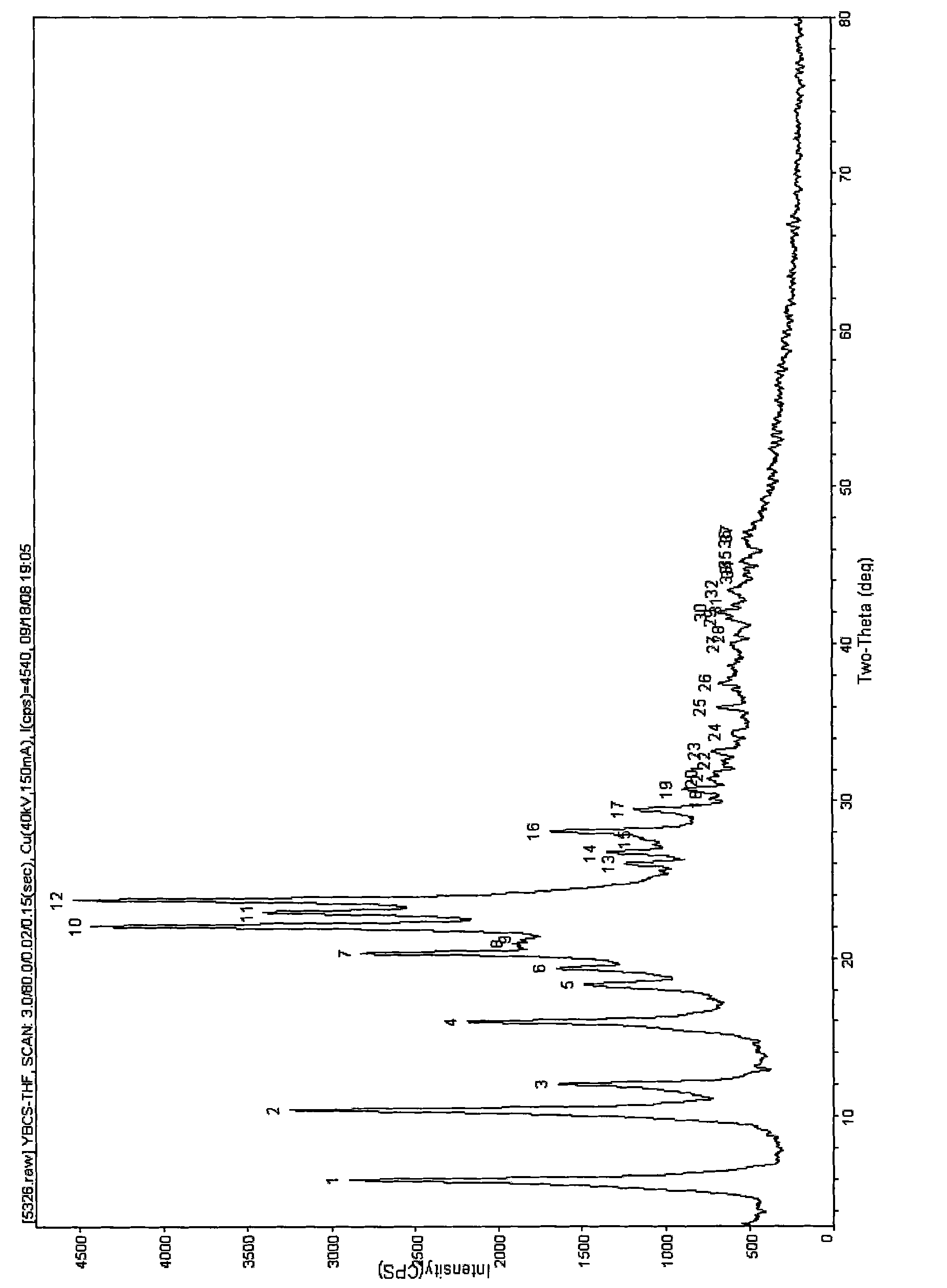
[0067] The chemical purity and crystalline purity of the sample of the B-type solid substance of petogenin obtained by one embodiment of the present invention are both greater than 90%. When powder X-ray diffraction is used to analyze and adopt CuKα radiation experimental conditions, the diffraction peak position is: 2-Theta value (°) or d value and diffraction peak relative intensity: peak height (Height%) or peak area (Area%) has the following characteristic peaks (see figure 1 ).
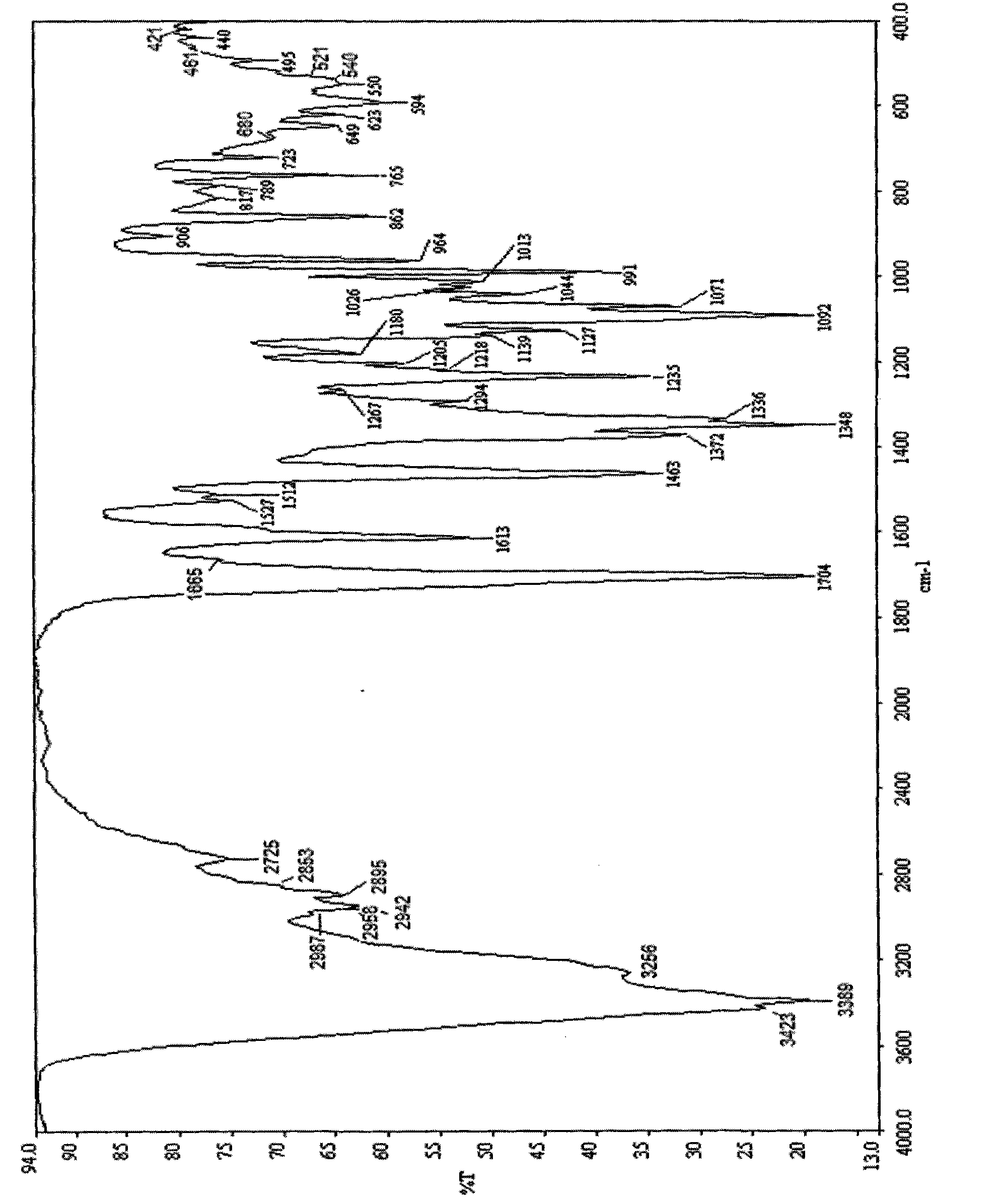
[0068] For the petracenin crystal B-type solid material obtained in one embo...
Embodiment 1
[0073] Preparation method 1 of petracenin crystal type C sample
[0074] Take 5g of petracenin sample, completely dissolve the petracenin sample with absolute ethanol at a normal temperature of 20°C, heat to a temperature of 40°C, and quickly evaporate the solvent under vacuum conditions to prepare the obtained petracenin 4.20g of plain crystal solid substance. HPLC purity 98.9%, recovery 84%.
[0075] The petracenin crystal C-type solid substance obtained by one embodiment of the present invention, the chemical purity and crystal form purity of its sample are both greater than 90%. When using powder X-ray diffraction analysis and using CuKα radiation experimental conditions, the diffraction peak position: 2-Theta value (°) or d value and diffraction peak relative intensity: peak height value (Height%) or peak area value (Area%) have following characteristic peak (see table 1 and Figure 4 ).
[0076] For the petracenin crystal C-type solid material obtained in one embodi...
Embodiment 2 to 8
[0080] Preparation method 2 to 8 of petracenin crystal type C sample
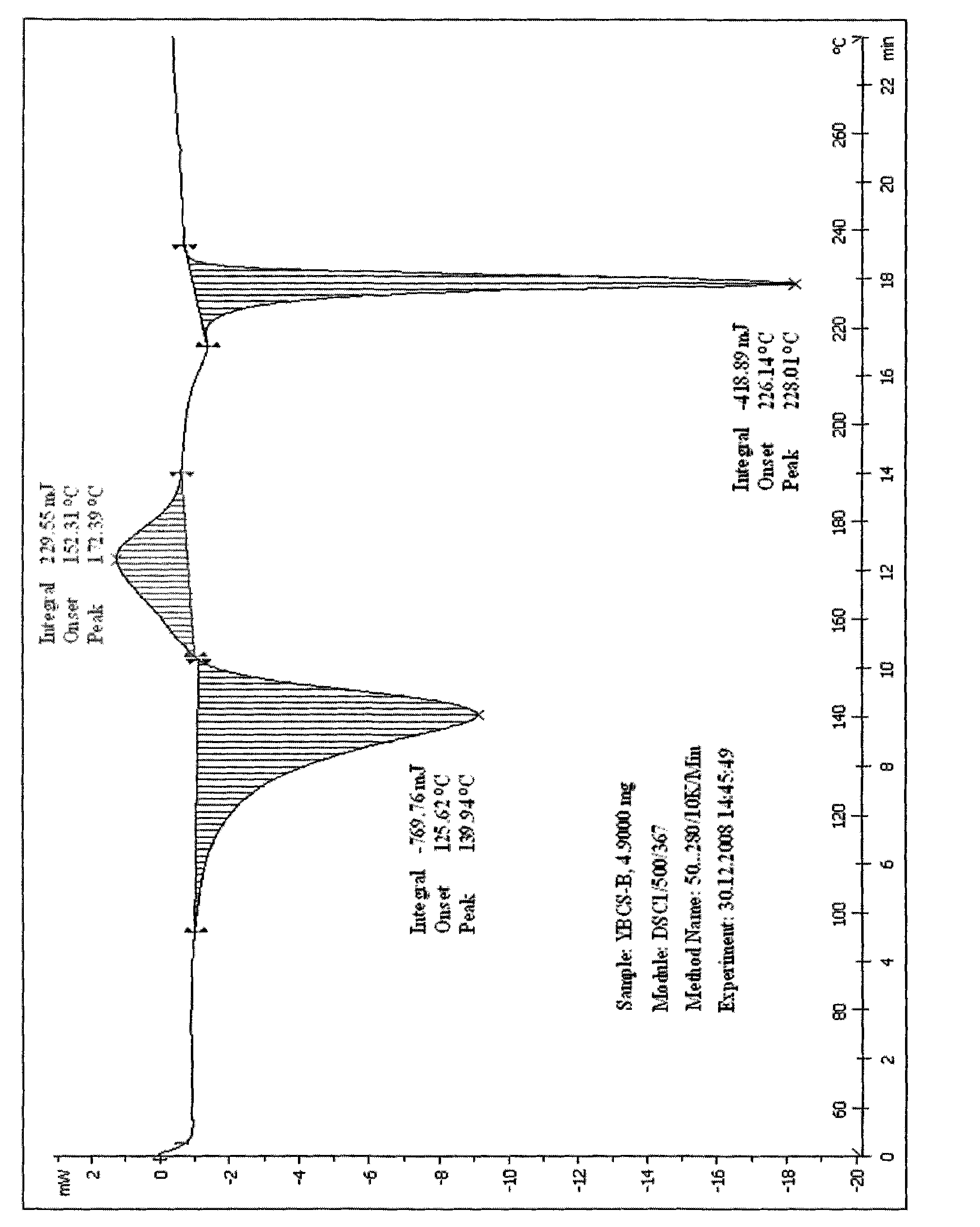
[0081] Referring to the preparation method of Example 1, DMF, dioxane, n-butanol, isopropanol, dichloromethane, ethyl acetate, chloroform or pyridine were used as solvents to obtain petracenin solids. The experimental results are shown in Table 2 below. Powder X-ray diffraction, IR, melting point determination and DSC analysis were carried out on the obtained crystals, which showed that the crystal form obtained was the crystal type C of petogenin.
[0082] The preparation result of table 2 petracenin crystal type C sample 1
[0083] Example
[0084] 8
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com