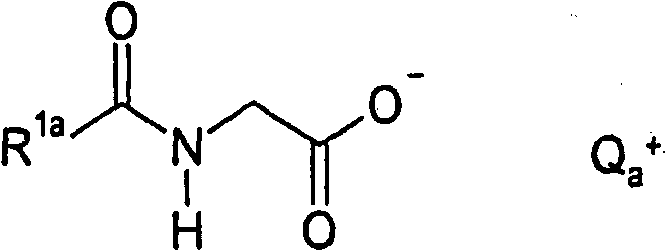
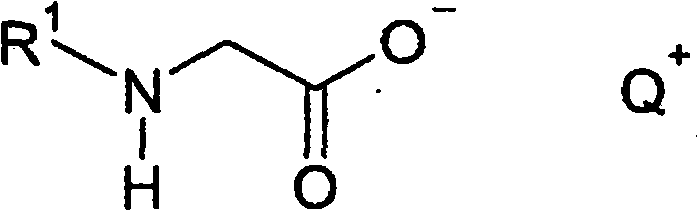
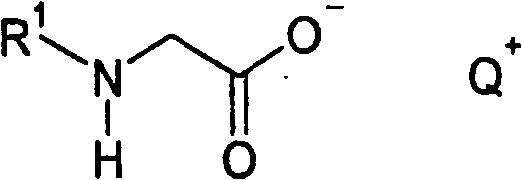
Method for producing acylglycinates
A technology of acyl glycinate and acyl, which is applied in the field of preparation of acyl glycinate, can solve complex problems and achieve cost-effective results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] 37.8 g (0.504 mol) of glycine were dissolved in 276 g of demineralized water with stirring, and the pH (current conditions) was adjusted to 12-13 with sodium hydroxide solution (33%). Subsequently, heating with stirring to 30-35° C., 106.4 g (0.478 mol) of cocoyl chloride (A) were metered in by cooling the reaction mixture at 30-35° C. within 6 hours. The pH was maintained at 12-13 by simultaneously metering in sodium hydroxide solution (33%). Towards the end of the metered addition of cocoyl chloride, the pH was lowered to 9.5-10.5. In order for the reaction to go to completion, it was stirred for an additional 2 hours at pH 9.5-10.5.
[0087] The resulting product has the following properties: liquid, milky white, dry residue (1 hour, 140°C): 31.0%, glycinate (HPLC): 0.6%, fatty acid salt (HPLC): 0.6%, viscosity (35°C): 756mPa • s, NaCl (titration): 5.3%, active ingredient content: 24.5%.
[0088] The weight of acyl glycinate in the composition according to the pre...
Embodiment 4
[0095] Example 4: Higher Glycine Excess
[0096] 37.8 g (0.504 mol) of glycine were dissolved in 250 g of demineralized water with stirring, and the pH (current conditions) was adjusted to 12-13 with sodium hydroxide solution (33%). Subsequently, heating was carried out to 30-35° C. with stirring, and 95.2 g (0.428 mol) of cocoyl chloride (A) were metered in at 30-35° C. within 6 hours while cooling the reaction mixture. The pH was maintained at 12-13 by simultaneously metering in sodium hydroxide solution (33%). Towards the end of the metered addition of cocoyl chloride, the pH was lowered to 9.5-10.5. In order for the reaction to go to completion, it was stirred for an additional 2 hours at pH 9.5-10.5.
[0097] The resulting product has the following properties: liquid, milky white, dry residue (1 hour, 140°C): 31.3%, glycinate (HPLC): 1.3%, fatty acid salt (HPLC): 0.2%, viscosity (35°C): 980mPa • s, NaCl (titration): 5.1%, active ingredient content: 24.7%.
Embodiment 5
[0098] Example 5: Higher concentration
[0099] 37.8 g (0.504 mol) of glycine were dissolved in 246 g of demineralized water with stirring, and the pH (current conditions) was adjusted to 12-13 with sodium hydroxide solution (33%). Subsequently, heating was carried out to 30-35° C. with stirring, and 106.4 g (0.478 mol) of cocoyl chloride (A) were metered in at 30-35° C. within 6 hours while cooling the reaction mixture. The pH was maintained at 12-13 by simultaneously metering in sodium hydroxide solution (33%). Towards the end of the metered addition of cocoyl chloride, the pH was lowered to 9.5-10.5. In order for the reaction to go to completion, it was stirred for an additional 2 hours at pH 9.5-10.5.
[0100] The resulting product has the following properties: liquid, milky white, dry residue (1 hour, 140°C): 32.7%, glycinate (HPLC): 0.8%, fatty acid salt (HPLC): 1.3%, viscosity (35°C): 4500mPa · s, NaCl (titration): 5.6%, active ingredient content: 25.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com