Rotundine crystalline C-type solid substance and preparation method as well as application
A solid substance, rotundine technology, applied in the field of medicine, can solve problems such as poor absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Preparation method 1 of crystalline rotundine sample:
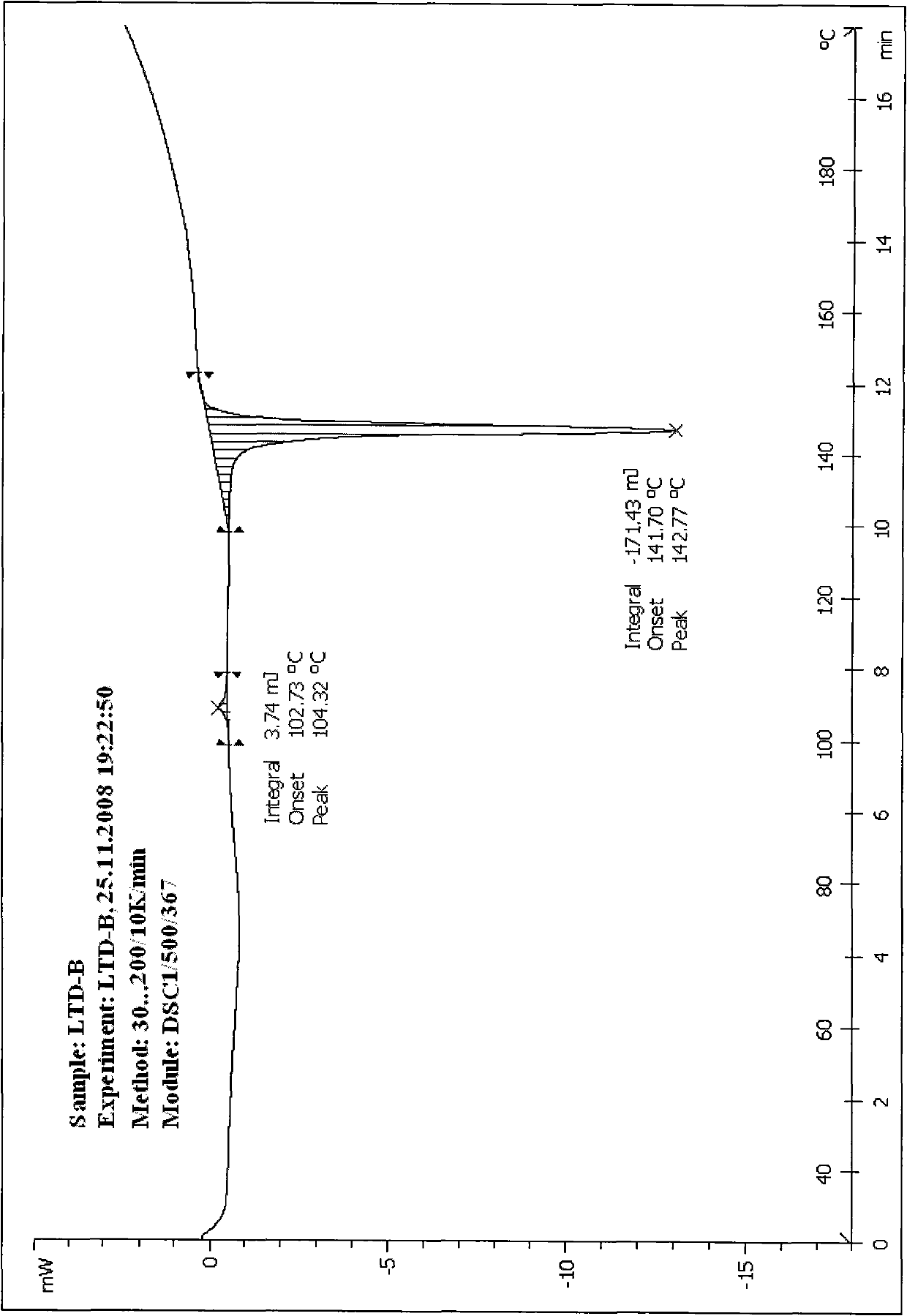
[0094] The preparation method of the crystal type B rotundine sample uses the crystal type A solid sample as the preparation raw material, and adopts the method of physical and mechanical lattice destruction and molecular rearrangement to crystallize to obtain the crystal type B solid material.
[0095] Preparation method 2 of crystalline rotundine sample:
[0096] The preparation method of the crystalline type B rotundine sample comprises firstly using a tetrahydrofuran solvent to completely dissolve the rotundine sample at a normal temperature of 15-30° C., and then rapidly preparing the crystalline type B rotundine solid substance by using a cold spray method.
Embodiment 2
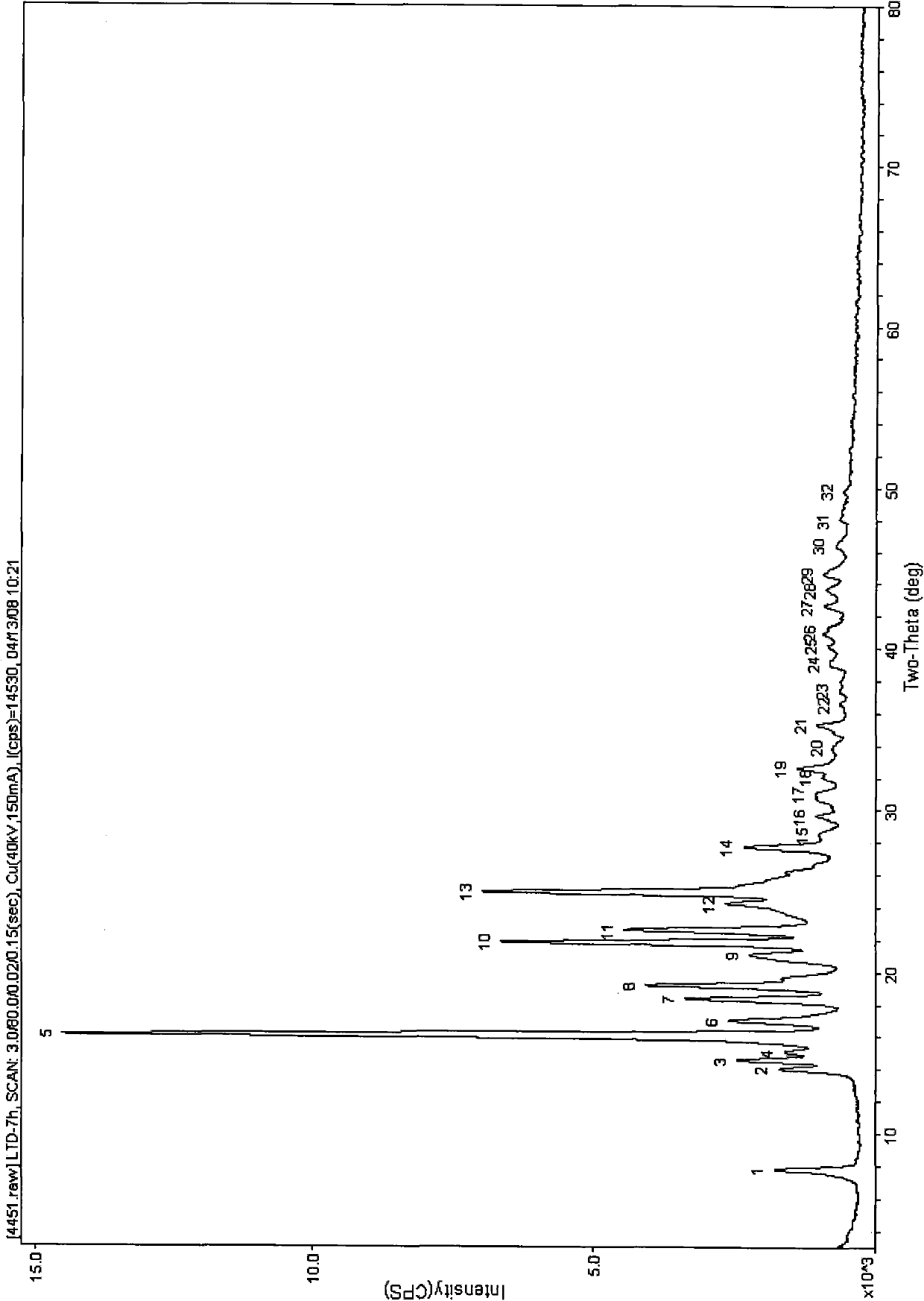
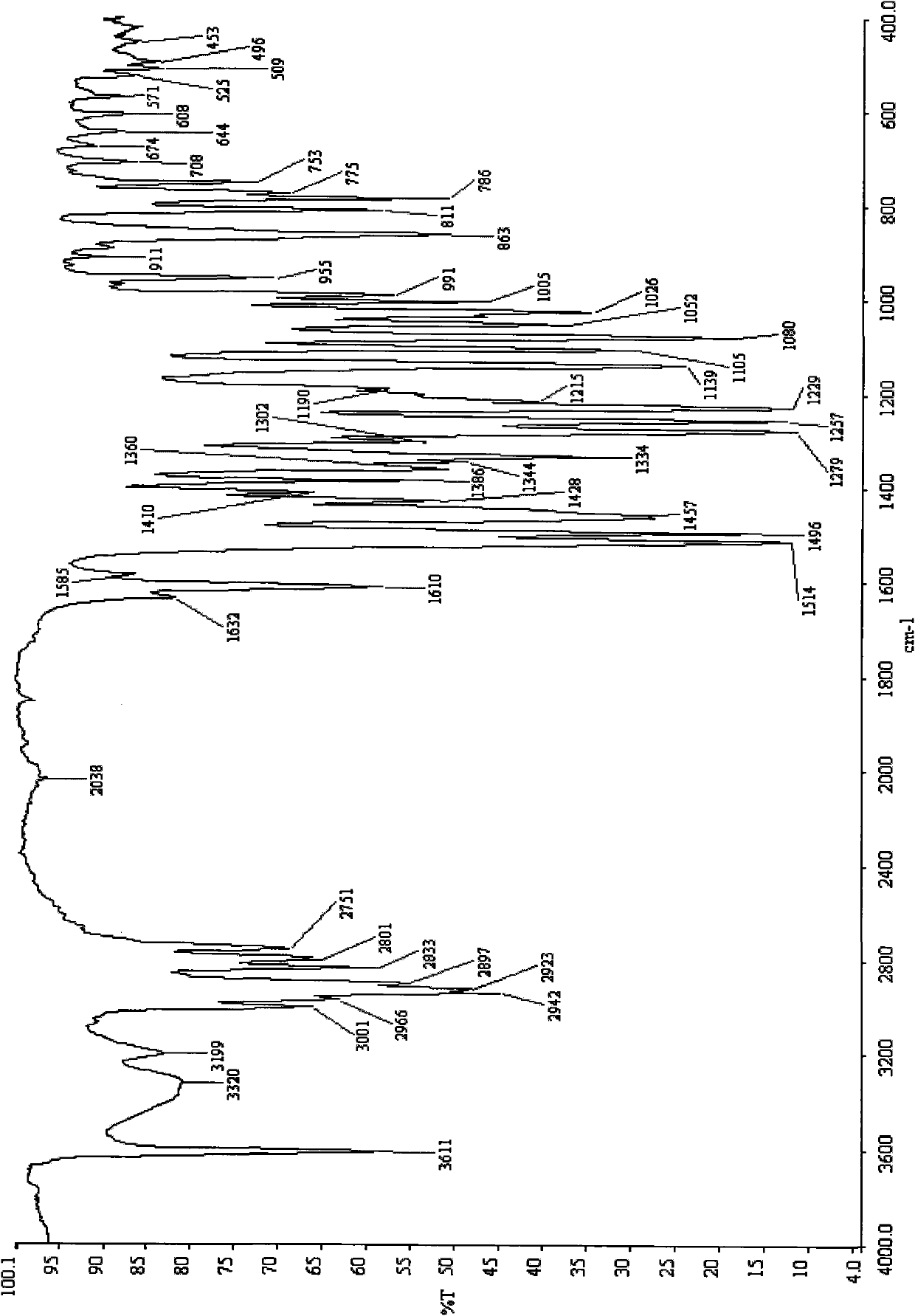
[0098] Crystalline C-type rotundine sample preparation method 1:
[0099] The preparation method of crystal C-type rotundine sample is to completely dissolve the rotundine sample with n-butanol solvent at room temperature of 15-30°C, and then use a rotary evaporator to quickly dissolve the solvent under vacuum at a temperature of 40°C. The obtained crystal C-type rotundine solid substance was prepared by distilling off.
[0100] Method 2 for the preparation of crystal C-type rotundine samples:
[0101] The preparation method of the crystalline type C rotundine sample is prepared by first dissolving the rotundine sample completely with a pyridine solvent at a normal temperature of 15-30°C, and then placing it under normal pressure at a temperature of 22°C to gradually volatilize the solvent. The crystalline type C rotundine solid substance.
Embodiment 3
[0103] The absorption characteristics and blood concentration characteristics of crystal B-type rotundine solid drug in rats:
[0104] Experimental animals: Wistar rats, male, body weight: 194.2±9.2g. purchased from the Institute of Experimental Animals, Chinese Academy of Medical Sciences.
[0105] Experimental method: Luotong fixed crystal type A, crystal type B. Prepare about 17mg / ml suspension with appropriate solution (physiological saline). Rats were reared under conventional feeding conditions, free to drink water, and after fasting for 12 hours, 200 mg / kg of drugs were given by intragastric administration. 0.083, 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 24 (h) after administration. About 0.3-0.5ml of blood was collected from the orbit, and centrifuged at 5000rpm for 15min. Take 150 μl of plasma, add 1ml of ethyl acetate, vortex for 3 minutes, centrifuge at 13400 rpm for 5 minutes, take 800 μl of organic layer, and evaporate to dryness with nitrogen. The resi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com