shRNA for hepatitis b virus and recombinant adeno-associated virus vector treating vector carrying same
A hepatitis B virus and virus gene technology, applied in the fields of molecular biology and biomedicine, can solve problems such as unsatisfactory treatment effect, and achieve the effects of long duration, stable inhibitory effect, and inhibition of replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis and sequence identification of DNA capable of transcribing shRNA.
[0038] According to the common parts of the three subtype sequences of HBV genotypes B, C, and D, the corresponding target sequence of HBV was selected by bioinformatics methods, and a DNA sequence capable of transcribing shRNA in vivo was designed for the target sequence. The DNA sequence was as follows:
[0039] Justice Chain:
[0040] 5′-CACCGCAGTTTACTAGTGCCATTTGTTTCAAGAGAACAAATGGCACTAGTAAACTGTTTT
[0041] TTG-3′
[0042] Antisense strand:
[0043] 5′-GATCCAAAAAACAGTTTACTAGTGCCATTTGTTCTCTTGAAACAAATGGCACTAGTAAAC
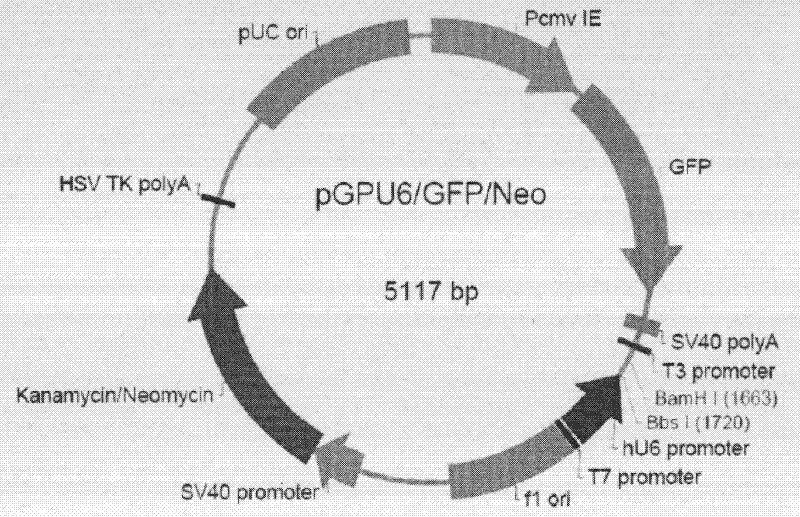
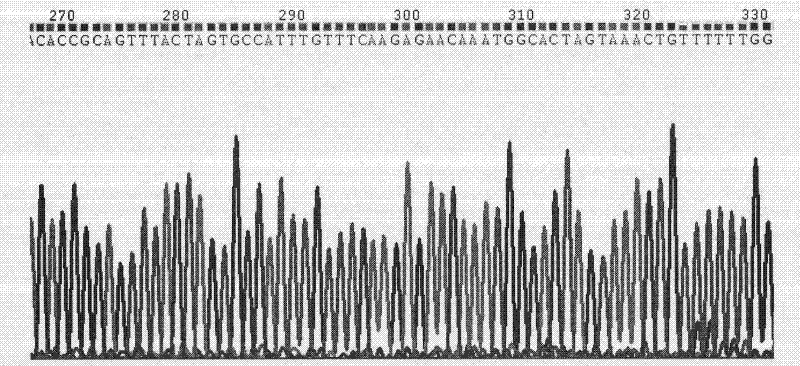
[0044] TGC-3′, a synthetic interference plasmid pGPU6-GFP-shRNA, was synthesized by Shanghai Gemma Pharmaceutical Technology Co., Ltd. and sequenced to identify the DNA sequence, such as figure 1 and 2 .
Embodiment 2
[0045] Example 2: Construction of recombinant adeno-associated virus backbone plasmid
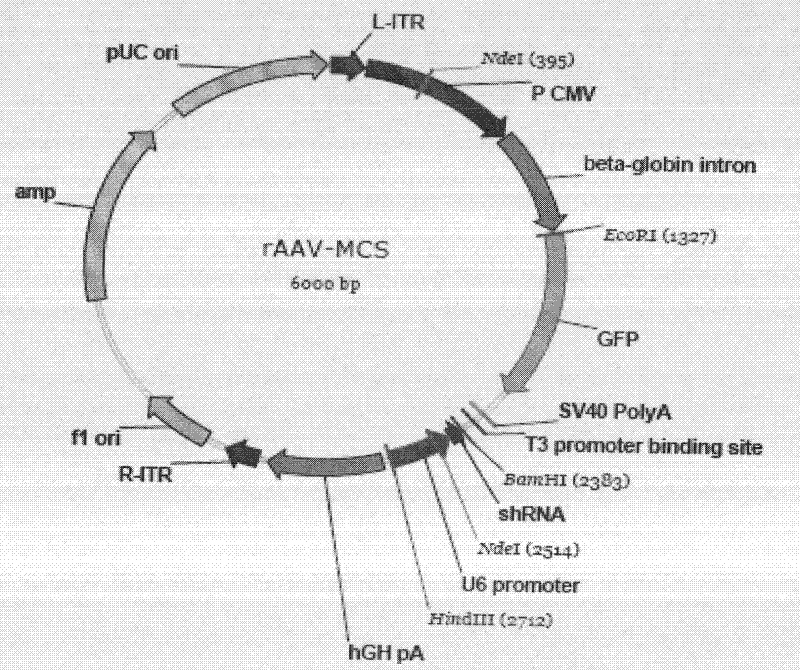
[0046] Using pGPU6-GFP-shRNA vector as template, 5′-GAATTCATGGTGAGCAAGGGCGAGGA-3′ and 5′-AAGCTTAAGGTCGGGCAGGAAGAGGG-3′ as upstream and downstream primers, amplify the GFP-shRNA-U6 fragment of plasmid pGPU6-GFP-shRNA, and introduce restriction Sites for endonucleases EcoR I and HindIII. Amplification conditions were: pre-denaturation at 94°C for 3 min, denaturation at 94°C for 30 sec, annealing at 65°C for 1 min, extension at 72°C for 2 min, a total of 30 cycles, and a final reaction at 72°C for 5 min. After the PCR product was recovered, it was inserted into the pGEM-T vector, and after the sequence identification was correct, it was cloned into the pAAV-MCS plasmid (Stratagene Company) with EcoR I and HindIII double enzyme digestion and sequenced (Shanghai Sangong) to identify positive recombinant clones. Named as the recombinant vector rAAV-shRNA-GFP, such as image 3 .
Embodiment 3
[0047] Example 3: Packaging of recombinant adeno-associated virus
[0048] will be 3×10 6 293T cells were inoculated in a culture dish with a diameter of 10 cm. When the confluence reached 70-80%, each 5-20 ug of rAAV-shRNA-GFP and pAAV-RC (Stratagene Company) and pHelper (Stratagene) three plasmids were co-transfected into 293T cells, and the pAAV-MCS plasmid was used as a negative control to package the pAAV empty virus, and the fresh medium was changed after 6 hours, and the morphological changes of the cells and the changes of the medium color were observed during the period. After continuing to culture for 66-72 hours, scrape the cells from the culture dish with a cell spatula and transfer them together with the culture medium to a 15ml centrifuge tube. The cell suspension was subjected to four back-and-forth 10min dry ice-ethanol baths and 37°C water baths, and vortexed evenly after each freeze-thaw. Centrifuge at 10,000 rpm for 10 min at room temperature, collect the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com