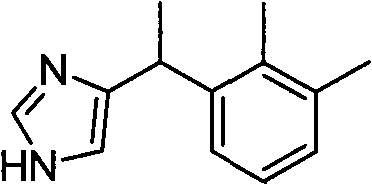
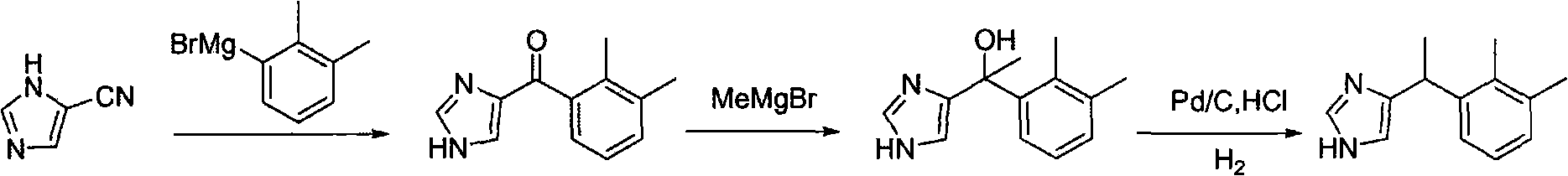Method for preparing dexmedetomidine
A technology of medetomidine and cyanimidazole, applied in the field of medicine, can solve the problems of high requirements on production equipment, high pressure on safety and environmental protection, unfavorable industrialized production, etc., and achieves low requirements on production equipment, reduced production costs, and easy industrialized production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
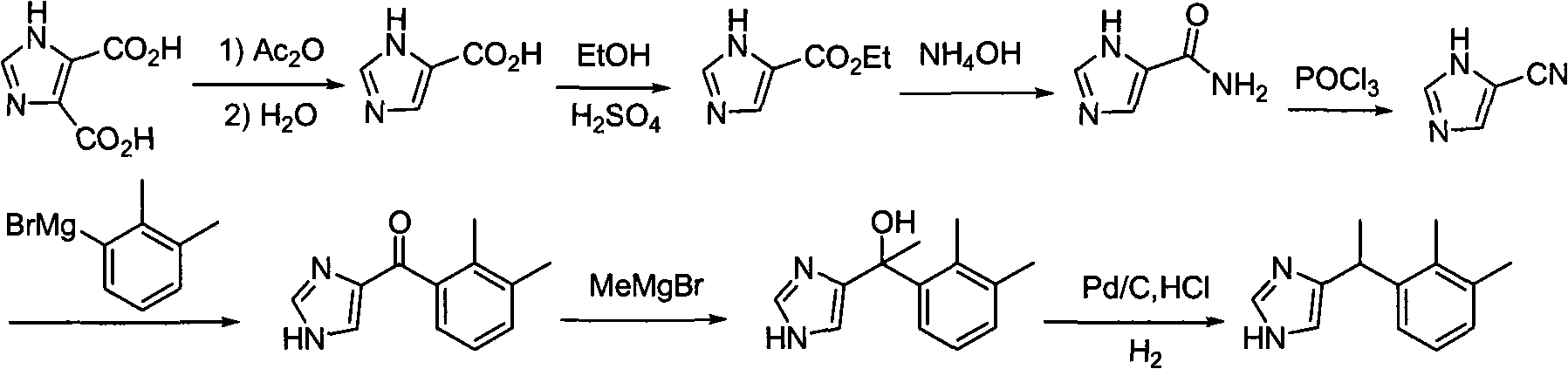
[0034] Medetomidine is prepared according to the following route:
[0035]
[0036] Preparation of 4-imidazolecarboxylic acid
[0037] Add 1.5 liters of acetic anhydride to 4,5-imidazoledicarboxylic acid (40 g, 0.2564 mol), stir and heat to the reflux temperature of acetic anhydride to react for 5 hours. Cool to room temperature. The resulting suspension was filtered to remove suspended solids, and the filtrate was concentrated to dryness under reduced pressure to obtain a brown-black viscous solid. Add 600 ml of water and stir at room temperature for 1 hour. Raise the temperature to 100°C and stir for 1 hour under heating, then cool down to 60°C, add 4 g of activated carbon and 600 ml of ethanol, and heat to reflux for 1 hour. When the temperature was lowered to 70°C, it was filtered with suction while it was hot to obtain a light yellow liquid, which was left to cool at room temperature for crystallization, and then further crystallized at 0°C for 12 hours, filtered wi...
Embodiment 2
[0052] In the same manner as in Example 1, ethyl 4-imidazolecarboxylate was obtained.
[0053] Preparation of 4-amidoimidazole
[0054] In the autoclave, add ethyl 4-imidazole formate (17.35 g, 0.124 mol) and 25 ml of concentrated ammonia (25%, density = 0.91 g / cm 3 ) (wherein ammonia is 0.335 mol) and ammonium chloride (0.85 g, 0.016 mol), and then reacted at 30° C. for 12 hours. The reaction solution was evaporated to dryness to obtain a brown solid, which was added with 100 ml of water, heated until the solid was dissolved, and left to cool at room temperature for crystallization. Further crystallization at 0° C. for 12 hours, suction filtration, and drying gave 12 g of 4-amidoimidazole with a yield of 88%.
[0055] Preparation of 4-cyanoimidazole
[0056] 4-amidoimidazole (7.4 g, 0.067 mol) and 16 g of thionyl chloride (0.134 mol) were reacted at 40° C. for 12 hours, and the thionyl chloride was distilled off under reduced pressure. 20 ml of ice water was added under a...
Embodiment 3
[0063] In the same manner as in Example 1, ethyl 4-imidazolecarboxylate was obtained.
[0064] Preparation of 4-amidoimidazole
[0065] In the autoclave, add 4-imidazole formic acid ethyl ester (17.35 grams, 0.124 mole), 900 milliliters of concentrated ammonia water (25%, density=0.91g / cm 3 ) (wherein ammonia is 12.044 mol) and ammonium chloride (6.5 g, 0.122 mol), and then reacted at 100° C. for 1 hour. The reaction solution was evaporated to dryness to obtain a brown solid, which was added with 50 ml of water, heated until the solid was dissolved, and left to cool at room temperature for crystallization. Further crystallization at 0° C. for 12 hours, suction filtration, and drying gave 9.4 g of 4-amidoimidazole with a yield of 85%.
[0066] Preparation of 4-cyanoimidazole
[0067] 4-amidoimidazole (7.4 g, 0.067 mol) and 400 g of thionyl chloride (3.35 mol) were reacted at 76° C. for 8 hours, and the thionyl chloride was distilled off under reduced pressure. 20 ml of ice ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com