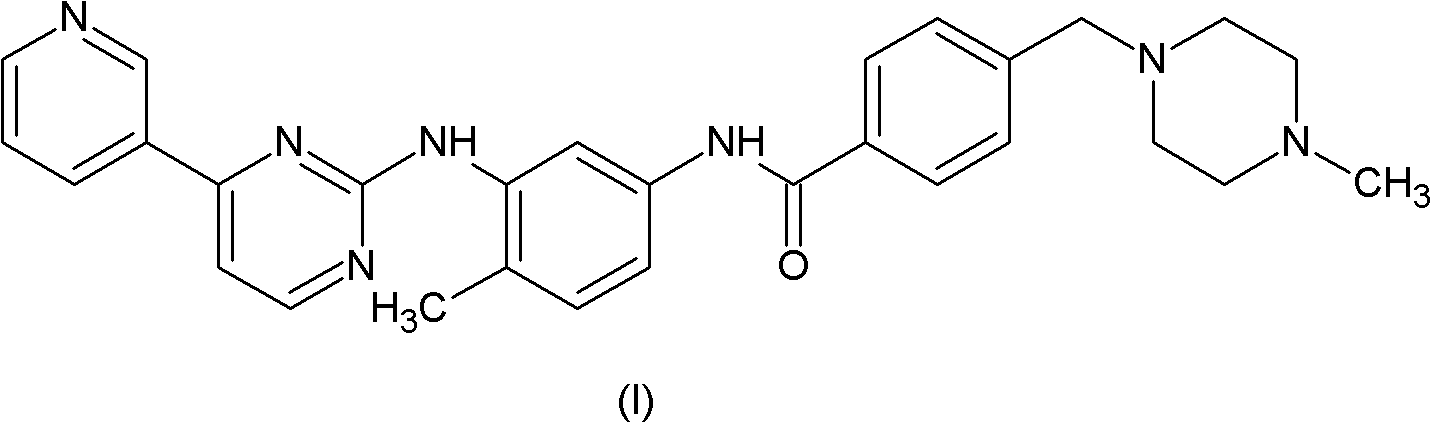
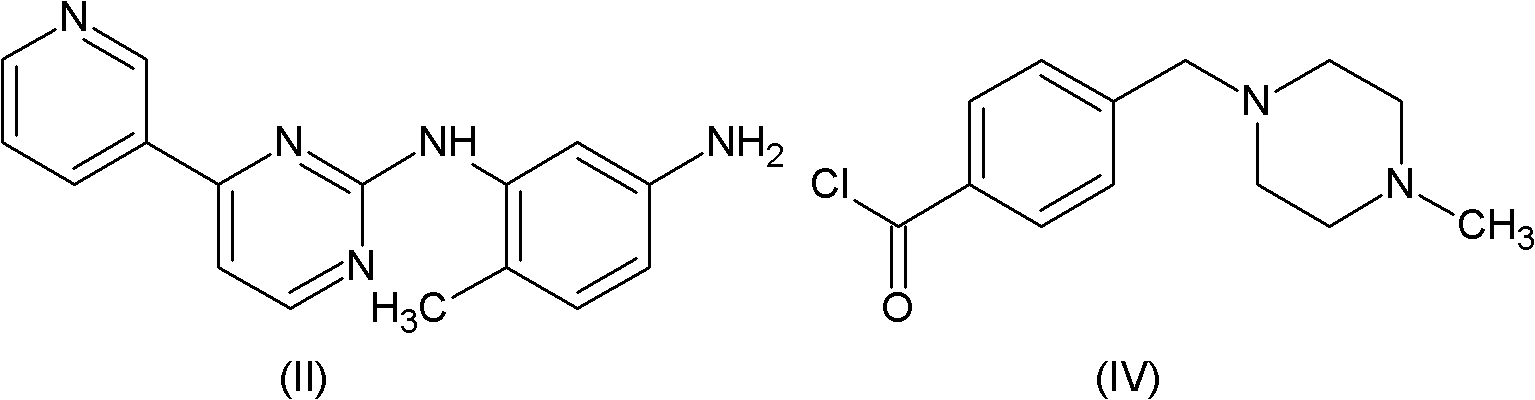
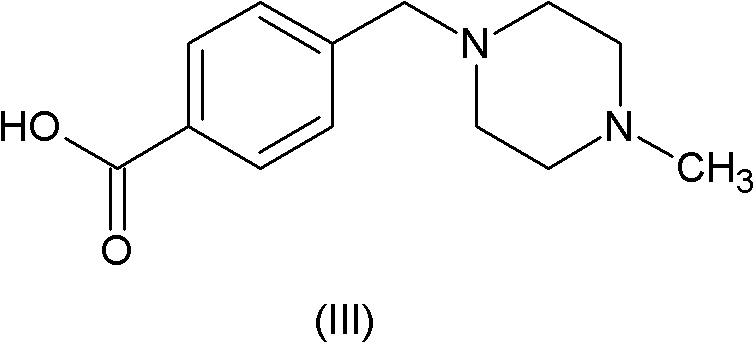
Method for preparing imatinib
A compound and structural formula technology, applied in the field of imatinib preparation, can solve the problem of low reaction yield, and achieve the effects of simple reaction steps, less environmental pollution, and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Magnetic stirring, a thermometer and a reflux condenser were installed in a 2000ml three-necked flask, and 27.7g (100mmol) of compound (II), 24.6g of compound (III), 0.5ml of pyridine and 450ml of N,N-dimethyl Acetamide was heated to 80-85°C, 32.6g of trimethyl phosphite was added dropwise within half an hour, and the reaction was continued for 2 hours at this temperature. Cool down to room temperature, add 450ml of water, extract with dichloromethane, spin dry, recrystallize with acetonitrile, and dry in vacuo to obtain 46.9g of imatinib (HPLC: 99.6%), with a yield of 95.0%.
Embodiment 2
[0034] Magnetic stirring, thermometer and reflux condenser are installed in the there-necked flask of 2000ml, add the imatinib intermediate (II) of 27.7g (100mmol), the imatinib intermediate (III) of 23.4g (100mmol), 0.5ml of pyridine and 500ml of N,N-dimethylformamide were heated to 50°C, 31.0g (100mmol) of triphenyl phosphite was added dropwise within 2 hours, and the reaction was continued at this temperature for 3 hours. Cool down to room temperature, add 500ml of water, extract with dichloromethane, spin dry, recrystallize with acetonitrile, and dry in vacuo to obtain 43.9g of imatinib (HPLC: 99.45%), with a yield of 89.0%.
Embodiment 3
[0036] In the there-necked flask of 2000ml, magnetic stirring, thermometer and reflux condenser are installed, add the imatinib intermediate (II) of 27.7g (100mmol), the imatinib intermediate (III) of 28.1g (120mmol), 0.1 ml of pyridine and 180 ml of N-methylpyrrolidone were heated to 90° C., 37.2 g (120 mmol) of triphenyl phosphite were added dropwise within 2 hours, and the reaction was continued at this temperature for 1.5 hours. Cool down to room temperature, add 180 ml of water, extract with dichloromethane, spin dry, recrystallize with acetonitrile, and dry in vacuo to obtain 40.0 g of imatinib (HPLC: 99.6%), with a yield of 81.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com