Method for improving pig nucleus transplantation efficiency
A technology of cell nucleus and donor cells, which is applied in the field of improving the efficiency of somatic cell nuclear transplantation, can solve problems such as not getting ideal results, and achieve the effect of improving efficiency and production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The mature culture of embodiment 1 porcine oocyte
[0024] The ovaries are collected from the slaughterhouse and transported back to the laboratory with 37°C normal saline, and the cumulus-oocyte complexes are collected from the antral follicles with a diameter of 3-5mm, and the oocytes with more than three complete layers of cumulus cells are selected under a solid microscope The cells were used for maturation culture; the culture conditions for maturation culture were 38.5°C, 5% carbon dioxide, 95% air gas environment, and saturated humidity; after 42 hours of maturation culture, 0.5% hyaluronidase was used to remove cumulus cells, and the obtained MII stage Oocytes, as nuclear transfer recipients.
Embodiment 2
[0025] Obtaining and culturing of pig fetal fibroblasts of embodiment 2
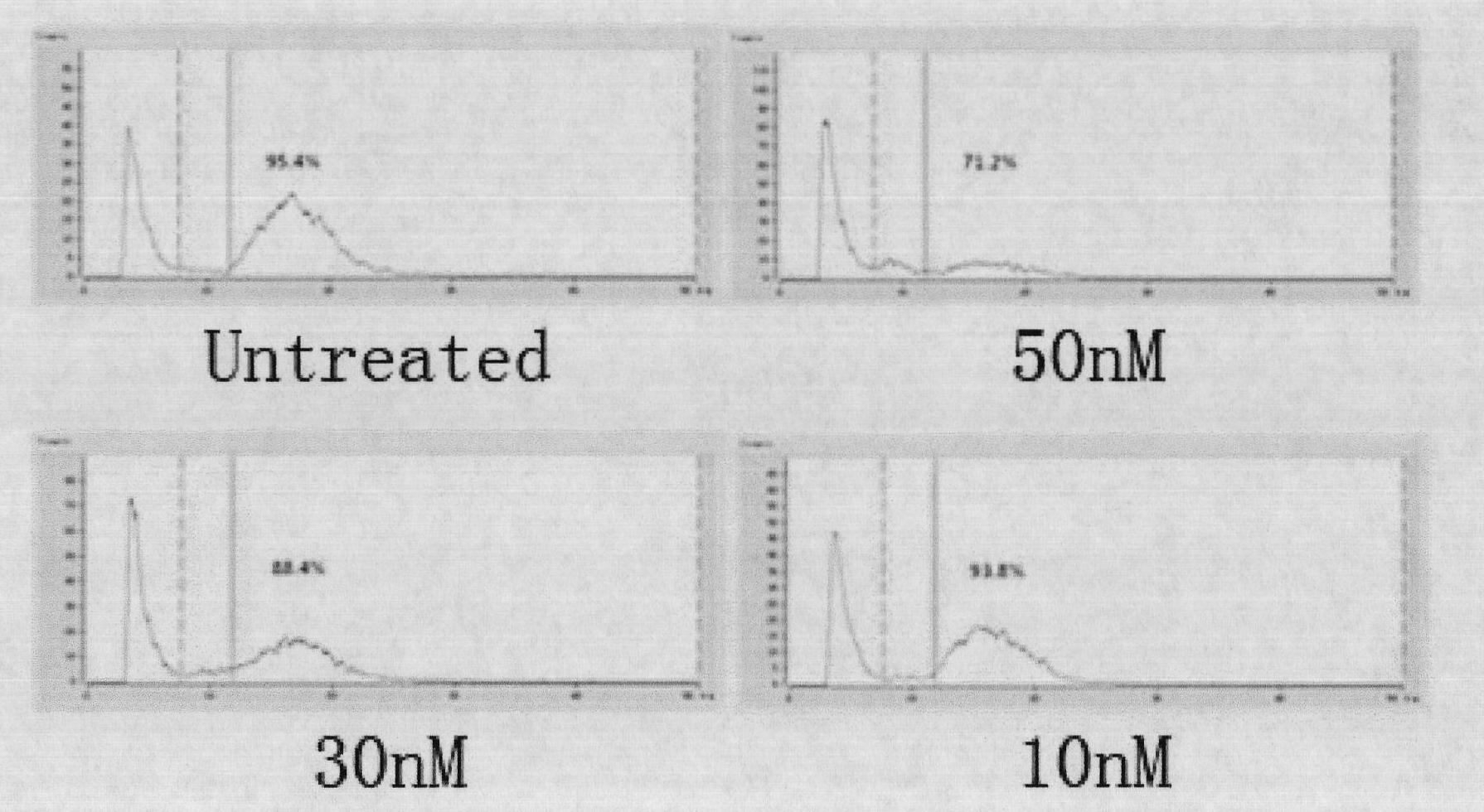
[0026] The ear tissue of 35-day-old pig fetus was taken aseptically, and the tissue cells were separated by conventional 0.25% trypsin (Gibco) digestion method, DMEM (Gibco) was added with 20% fetal bovine serum for primary culture, and DMEM was added with 10% fetal bovine serum for subculture , the culture condition is 38.5°C, 5% carbon dioxide, 95% air gas environment, moderately saturated; 3-5 passages of contact-inhibited fetal fibroblasts are used for nuclear transfer, and 50, 30, and 10 nM CalyculinA are used respectively before nuclear transfer (sigma) Fetal fibroblasts were pretreated for 30, 20, and 10 min, and then digested with 0.25% trypsin into a single suspension cell, which was used as a nuclear transfer donor.
Embodiment 3
[0027] Example 3 Somatic cell nuclear transfer
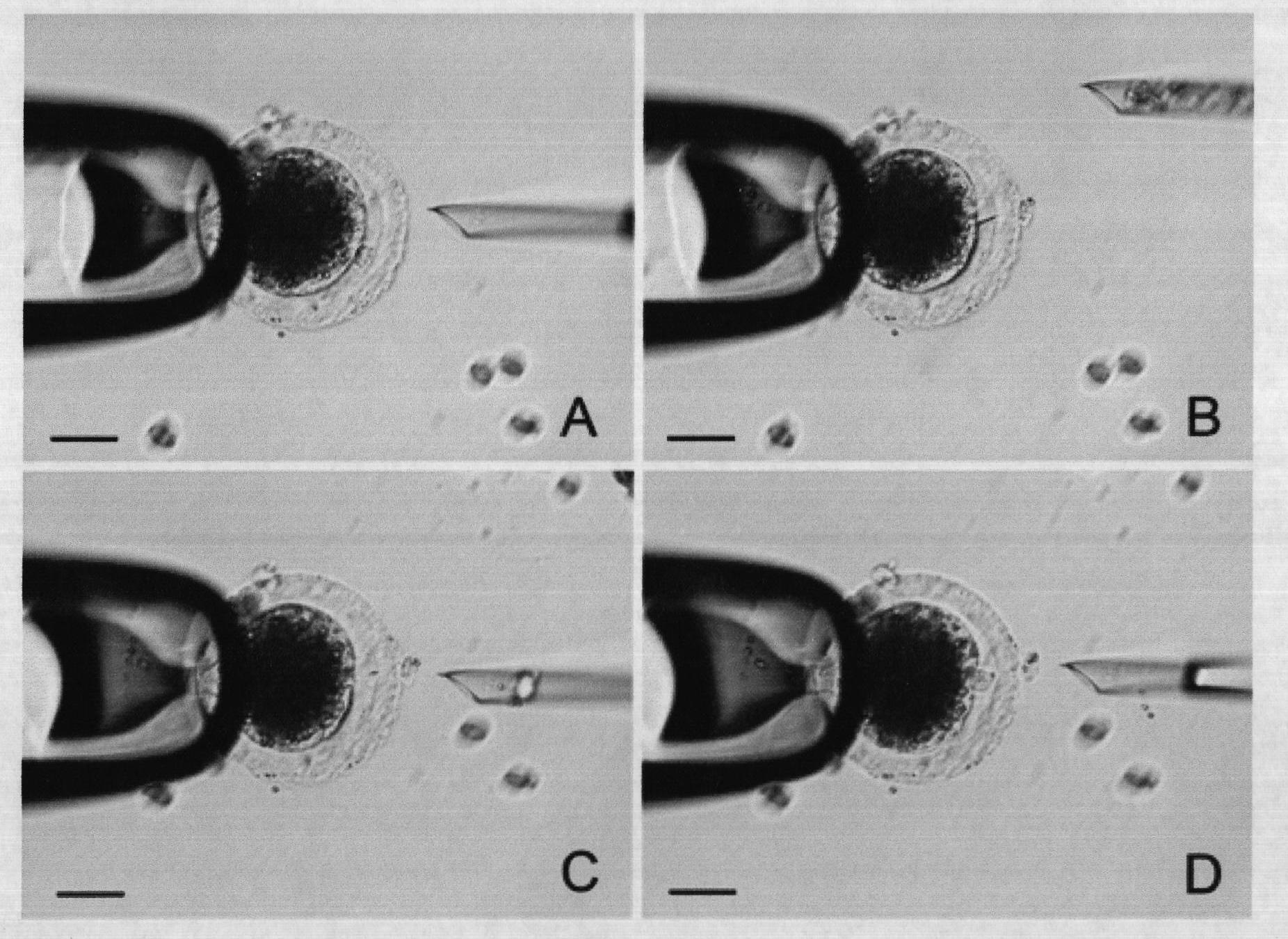
[0028] Porcine somatic cell nuclear transfer was performed by the fusion method ( figure 1), the process is as follows: the spindle body and the first polar body of the MII egg were removed by blind suction, the somatic cells were injected under the zona pellucida, and made in close contact with the oocyte plasma membrane, and DC electric shock (1.2kv / cm, 30μs, 2 subpulse) to induce fusion of somatic cells and enucleated oocytoplasm to form reconstituted embryos and activate them, and culture them in PZM-3 culture medium under the culture conditions of 38.5°C, 5% CO2, and saturated humidity. The cleavage rate was calculated after 48 hours of culture, and the blastocyst rate was calculated after 146 hours. 10mg / L Hoechst 33342 was used to stain blastocysts for 5 minutes, and the number of blastocyst cells was observed under a fluorescent microscope; the tools, liquids, and instruments used were as follows: the inner diameter of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com