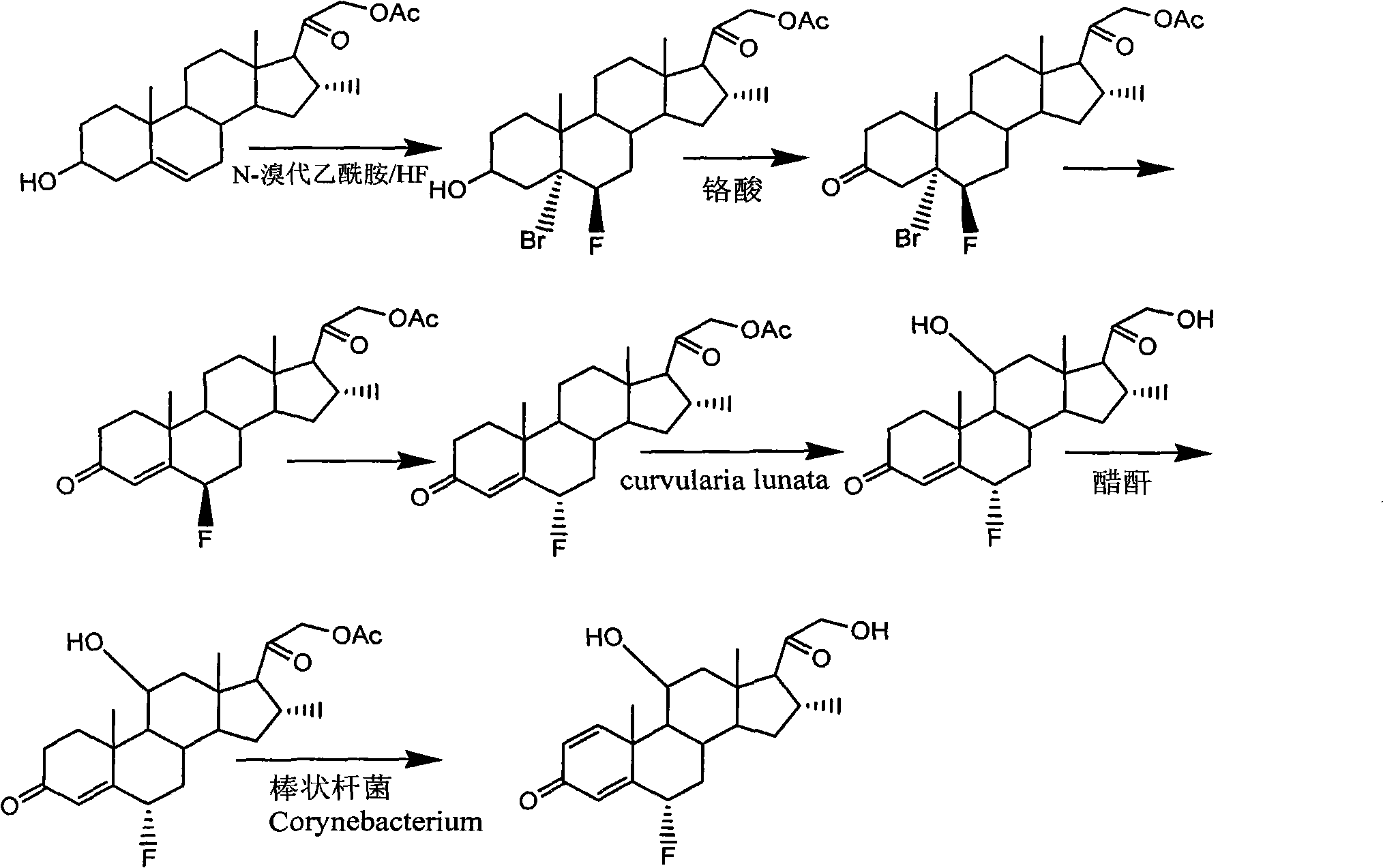
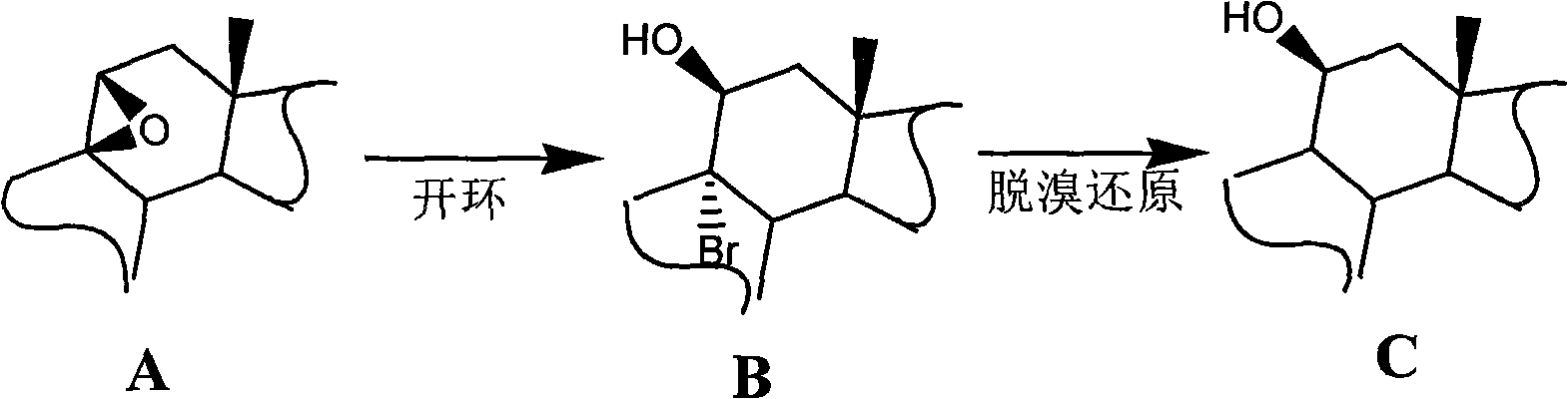
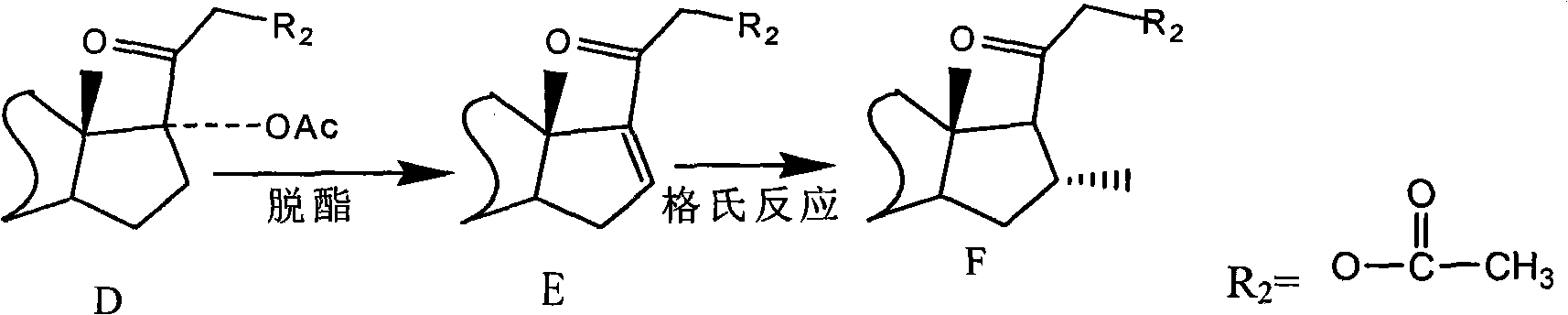
Preparation of fluorine-containing steroid hormone
A technology of fluoclon and its compounds, which is applied in the field of preparation of fluoclon and its esters, and can solve the problems of low production efficiency, low yield, unfavorable industrialized production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of embodiment 1 Fluocoron and Fluocoron 21-pivalate
[0072] Starting from 6α-fluoro-9,11-epoxy-17,21-dihydroxy-pregna-1,4-diene-3,20-dione-17,21-diacetate (I) , followed by 9, 11-epoxy ring opening, 9-position debromination reduction, 17-position deesterification to generate 16, 17-position double bonds, Grignard reaction 16-position methyl, get fluocorone 21-acetate, after hydrolysis Fluorocolon is obtained, and Fluorocorone is esterified to obtain the 21-position pivalate of Fluorocorone.
[0073] The reaction scheme is as follows:
[0074]
[0075] (1.1) 6α-fluoro-9α-bromo-11,17,21-trihydroxy-pregna-1,4-diene-3,20-dione-17,21-diacetate (1-II) preparation
[0076] 10g (I) and 40ml of acetic acid are dropped into the reaction flask under stirring, cooled to 10°C, then 10ml of hydrobromic acid is added dropwise in the reaction flask, monitored by thin-layer chromatography until no raw material is reacted, and the reaction time is 0.5 hour. The reacti...
Embodiment 2
[0087] The preparation of embodiment 2 fluoclon and fluoclon 21-butyrate
[0088] The reaction starts with 6α-fluoro-9,11-epoxy-17,21-dihydroxy-pregna-1,4-diene-3,20-dione-17,21-diacetate (I) 17-position deesterification to generate 16, 17-position double bond, 9, 11-epoxy ring opening, 9-position debromination reduction, 16, 17-position double bond through Grignard reaction, 16-position methyl, 21- Acetate is hydrolyzed to obtain fluoclon, and then esterified to obtain fluoclon 21-butyrate.
[0089]
[0090] (2.1) Preparation of 6α-fluoro-9,11-epoxy-21-hydroxy-pregna-1,4,16-triene-3,20-dione-21-acetate (2-II)
[0091] Put 10g of compound (I), 70ml of dimethylformamide, and 7g of potassium acetate into the reaction flask in turn, feed nitrogen gas, stir, and react at 125°C. Monitor with thin-layer chromatography until the reaction is complete. The reaction time is 1 hour, and then cooled When the temperature was below 40°C, the reaction solution was poured into 50 times i...
Embodiment 3
[0102] The preparation of embodiment 3 fluoclon and fluoclon 21-propionate
[0103] Starting from 6α-fluoro-9,11-epoxy-17,21-dihydroxy-pregna-1,4-diene-3,20-dione-17,21-diacetate (I) , followed by 9, 11-epoxy ring opening, 17-position deesterification to generate 16, 17-position double bond, 9-position debromination reduction, Grignard reaction 16-position methyl, get fluocorone 21-acetate, after hydrolysis Fluoroclon is obtained, and then esterified to obtain Fluoroclon 21-propionate.
[0104] The reaction scheme is as follows:
[0105]
[0106]
[0107] (3.1) 6α-fluoro-9α-bromo-11,17,21-trihydroxy-pregna-1,4-diene-3,20-dione-17,21-diacetate (3-II) preparation
[0108] Put 10g of compound (I) and 40ml of acetic acid into the reaction flask under stirring, the temperature is controlled at 25°C, then 12ml of hydrobromic acid is added dropwise into the reaction flask, and the reaction is completed by thin-layer chromatography monitoring until there is no raw material. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com